orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
Tempo:2024-01-12
1、Introduction
Today, through the study of empagliflozin peroxide impurities, we hope to provide assistance to clients in researching peroxide impurities in SGLT-2 inhibitor - gliflozin compounds. The two brightest stars among SGLT-2 inhibitors are empagliflozin and dapagliflozin. According to incomplete statistics, Boehringer Ingelheim's empagliflozin sales exceeded $8.2 billion in 2022, and AstraZeneca's dapagliflozin also sold nearly $4.4 billion in 2022. Information on the SGLT-2 inhibitor compound series is shown in Figure 1:

Figure 1: SGLT-2 Inhibitor Series Compound Information
2、Hotspot Introduction of Empagliflozin
Empagliflozin is currently available as a single agent and in combination (empagliflozin/metformin), so there are many companies engaged in generic R&D. According to Zhenqiang's internal data, over 45 companies purchased empagliflozin impurities from us in 2023 alone. Currently, the QCS website has cataloged a total of 94 empagliflozin impurities (scan the QR code at the end to view all impurity lists). This article will introduce the detection and research work conducted by our center on empagliflozin peroxides, referencing the Empagliflozin Tablets imported drug registration standard (Standard No. JX20150247) (empagliflozin peroxide structural information is shown in Figure 2).
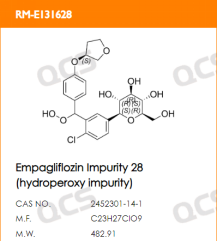
Figure 2: Empagliflozin Peroxide Structural Information
3、Peroxide Chromatographic Results and Structural Verification
According to the related substances method in the Empagliflozin Tablets imported drug registration standard (Standard No. JX20150247), our center first conducted HPLC qualitative positioning research on this peroxide impurity. The chromatographic conditions referred to the related substances method in the standard (see Figure 3).

Figure 3: Related Substances Method from the Tablet Imported Registration Standard
Referencing this method, our center tested the laboratory-synthesized empagliflozin peroxide. The mixed injection results of this peroxide impurity with empagliflozin API are shown in Figure 5, and the single injection chromatogram of the peroxide impurity is shown in Figure 4.

Figure 4: Empagliflozin Peroxide Detection Chromatogram
Mixed injection data of empagliflozin and empagliflozin peroxide is shown in Figure 5:

Figure 5: Mixed Sample Chromatogram of Empagliflozin and Its Peroxide
In the above chromatographic results, empagliflozin has a retention time of 7.7 minutes, and the peroxide impurity appears as a double peak at 3.6-3.8 minutes. Since the peroxide impurity is a mixture of a pair of diastereomers (structural formula see Figure 6, key chiral centers marked in red), the imported registration standard conditions provide some separation effect for this structure, resulting in the double peak.

Figure 6: Diagram of Chiral Carbon Positions Causing the Double Peak
The QCS R&D Center confirmed this sample using Liquid Chromatography-Mass Spectrometry (LCMS). Through method optimization, the double peak result of the empagliflozin peroxide impurity sample could be reproduced in LCMS (Figure 7). Comparison of the MS for the two chromatographic peaks (Figures 8 & 9) verified this conclusion.

Figure 7: LC-MS Chromatogram of the Peroxide Double Peaks

Figure 8: MS Results for the Chromatographic Peak at RT=38.272 min (left) and RT=40.275 min (right) in the Peroxide LC-MS Chromatogram (Mode: Positive Ion)

Figure 9: MS Results for the Chromatographic Peak at RT=38.272 min (left) and RT=40.275 min (right) in the Peroxide LC-MS Chromatogram (Mode: Negative Ion)
<span style="color: rgb(15, 17, 21); font-family: quote-cjk-patch, Inter, system-ui, -apple-system, BlinkMacSystemFont, "Segoe UI", Roboto, Oxygen, Ubuntu, Cantarell, "Open Sans", "Helvetica Neue", sans-
1、Introduction
Today, through the study of empagliflozin peroxide impurities, we hope to provide assistance to clients in researching peroxide impurities in SGLT-2 inhibitor - gliflozin compounds. The two brightest stars among SGLT-2 inhibitors are empagliflozin and dapagliflozin. According to incomplete statistics, Boehringer Ingelheim's empagliflozin sales exceeded $8.2 billion in 2022, and AstraZeneca's dapagliflozin also sold nearly $4.4 billion in 2022. Information on the SGLT-2 inhibitor compound series is shown in Figure 1:

Figure 1: SGLT-2 Inhibitor Series Compound Information
2、Hotspot Introduction of Empagliflozin
Empagliflozin is currently available as a single agent and in combination (empagliflozin/metformin), so there are many companies engaged in generic R&D. According to Zhenqiang's internal data, over 45 companies purchased empagliflozin impurities from us in 2023 alone. Currently, the QCS website has cataloged a total of 94 empagliflozin impurities (scan the QR code at the end to view all impurity lists). This article will introduce the detection and research work conducted by our center on empagliflozin peroxides, referencing the Empagliflozin Tablets imported drug registration standard (Standard No. JX20150247) (empagliflozin peroxide structural information is shown in Figure 2).

Figure 2: Empagliflozin Peroxide Structural Information
3、Peroxide Chromatographic Results and Structural Verification
According to the related substances method in the Empagliflozin Tablets imported drug registration standard (Standard No. JX20150247), our center first conducted HPLC qualitative positioning research on this peroxide impurity. The chromatographic conditions referred to the related substances method in the standard (see Figure 3).

Figure 3: Related Substances Method from the Tablet Imported Registration Standard
Referencing this method, our center tested the laboratory-synthesized empagliflozin peroxide. The mixed injection results of this peroxide impurity with empagliflozin API are shown in Figure 5, and the single injection chromatogram of the peroxide impurity is shown in Figure 4.

Figure 4: Empagliflozin Peroxide Detection Chromatogram
Mixed injection data of empagliflozin and empagliflozin peroxide is shown in Figure 5:

Figure 5: Mixed Sample Chromatogram of Empagliflozin and Its Peroxide
In the above chromatographic results, empagliflozin has a retention time of 7.7 minutes, and the peroxide impurity appears as a double peak at 3.6-3.8 minutes. Since the peroxide impurity is a mixture of a pair of diastereomers (structural formula see Figure 6, key chiral centers marked in red), the imported registration standard conditions provide some separation effect for this structure, resulting in the double peak.

Figure 6: Diagram of Chiral Carbon Positions Causing the Double Peak
The QCS R&D Center confirmed this sample using Liquid Chromatography-Mass Spectrometry (LCMS). Through method optimization, the double peak result of the empagliflozin peroxide impurity sample could be reproduced in LCMS (Figure 7). Comparison of the MS for the two chromatographic peaks (Figures 8 & 9) verified this conclusion.

Figure 7: LC-MS Chromatogram of the Peroxide Double Peaks

Figure 8: MS Results for the Chromatographic Peak at RT=38.272 min (left) and RT=40.275 min (right) in the Peroxide LC-MS Chromatogram (Mode: Positive Ion)

Figure 9: MS Results for the Chromatographic Peak at RT=38.272 min (left) and RT=40.275 min (right) in the Peroxide LC-MS Chromatogram (Mode: Negative Ion)
<span style="color: rgb(15, 17, 21); font-family: quote-cjk-patch, Inter, system-ui, -apple-system, BlinkMacSystemFont, "Segoe UI", Roboto, Oxygen, Ubuntu, Cantarell, "Open Sans", "Helvetica Neue", sans-
Junte-se à nossa lista de e-mails
Inscreva-se para receber atualizações sobre novos produtos, promoções e recursos!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*Todos os produtos desta empresa são destinados apenas para pesquisa científica.

*Todos os produtos desta empresa são destinados apenas para pesquisa científica.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号