orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
Tempo:2025-01-08
Attention! It has been proven that dihydropyridine calcium channel blockers (CCBs) cannot generate the corresponding nitrosamine structural impurities, and this conclusion has been accepted by the FDA.
Recently, a person claiming to be from Glenmark Pharmaceuticals in India stated that the company has confirmed that drugs ending with "-dipine" cannot generate the corresponding nitrosamine structural impurities, and this conclusion has been accepted by the FDA.
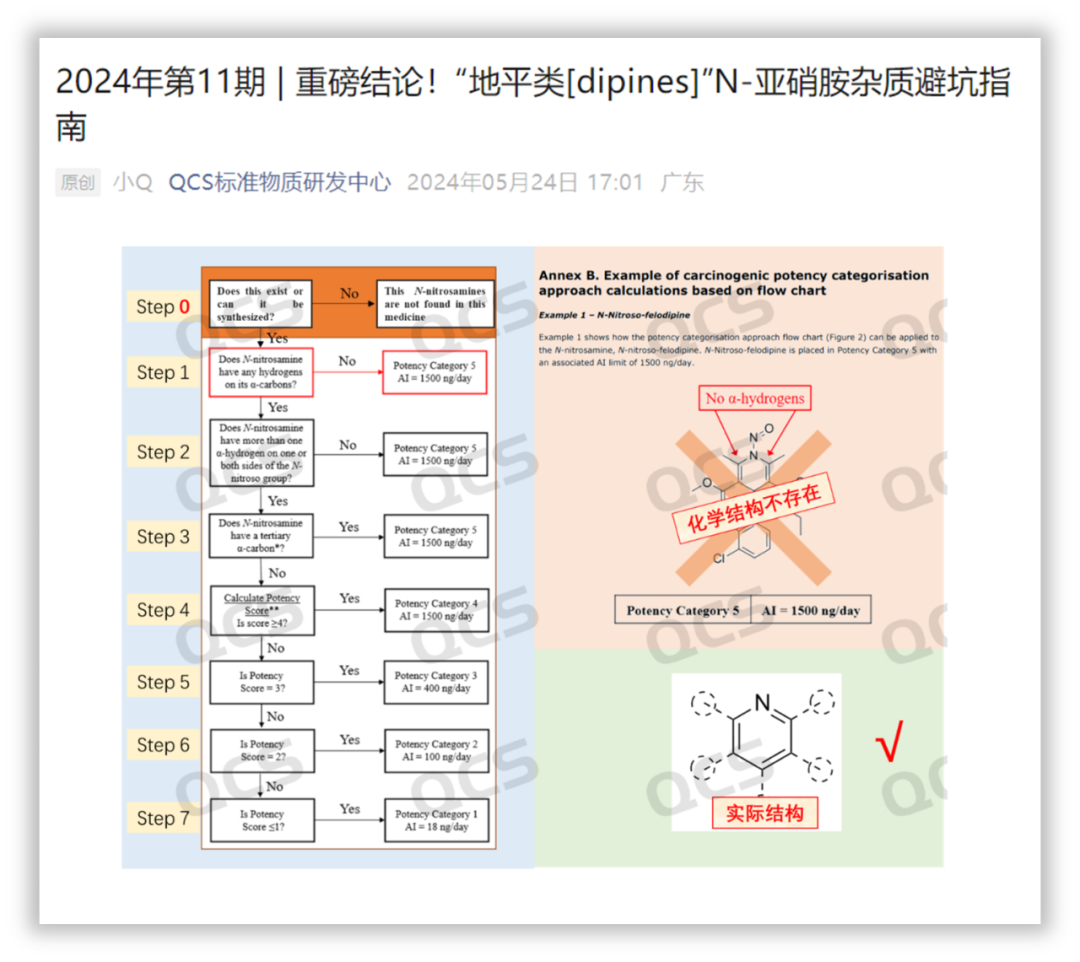
Figure 1: Screenshot of 2024
Earlier, in the 11th issue of the QCS Reference Material R & D Center's WeChat推文 in 2024, an article titled "Issue 11, 2024 | Groundbreaking Conclusion! A Guide to Avoiding Pitfalls with N - Nitrosamine Impurities in 'Dipines'" was published. In the article, based on the experiences of multiple self - developed projects, we reached the conclusion that nitrosamine impurities in dipines do not exist. Recently, we learned that a pharmaceutical company in India has submitted a declaration regarding the nitrosamine impurities in its dipine drugs according to this method, and it has been accepted by the FDA.
Recently, in the USP Nitrosamine Forum, a user named "Dileep5571" posted replies in two discussion groups, namely "Discussion on Nitrosamine Nicardipine Impurities" and "N - Nitrosamine Impurities Listed by the FDA and EMA: N - Nitrosoamlodipine and N - Nitrosfelodipine Cannot Be Synthesized". In the replies, the user stated that their company has confirmed that the N - nitroso impurities of dipine drugs cannot be synthesized, and they have submitted a declaration based on this conclusion, which has been accepted by the FDA.
Meanwhile, other users on the forum reported that the so - called dipine N - nitrosamines provided by multiple suppliers from different countries were all proven to be mislabeled products. They were identified as aromatized impurities rather than N - nitrosamine impurities. The user information indicates that the post was from Glenmark Pharmaceuticals Limited. Founded in 1977, it is one of the leading multinational pharmaceutical companies in India.
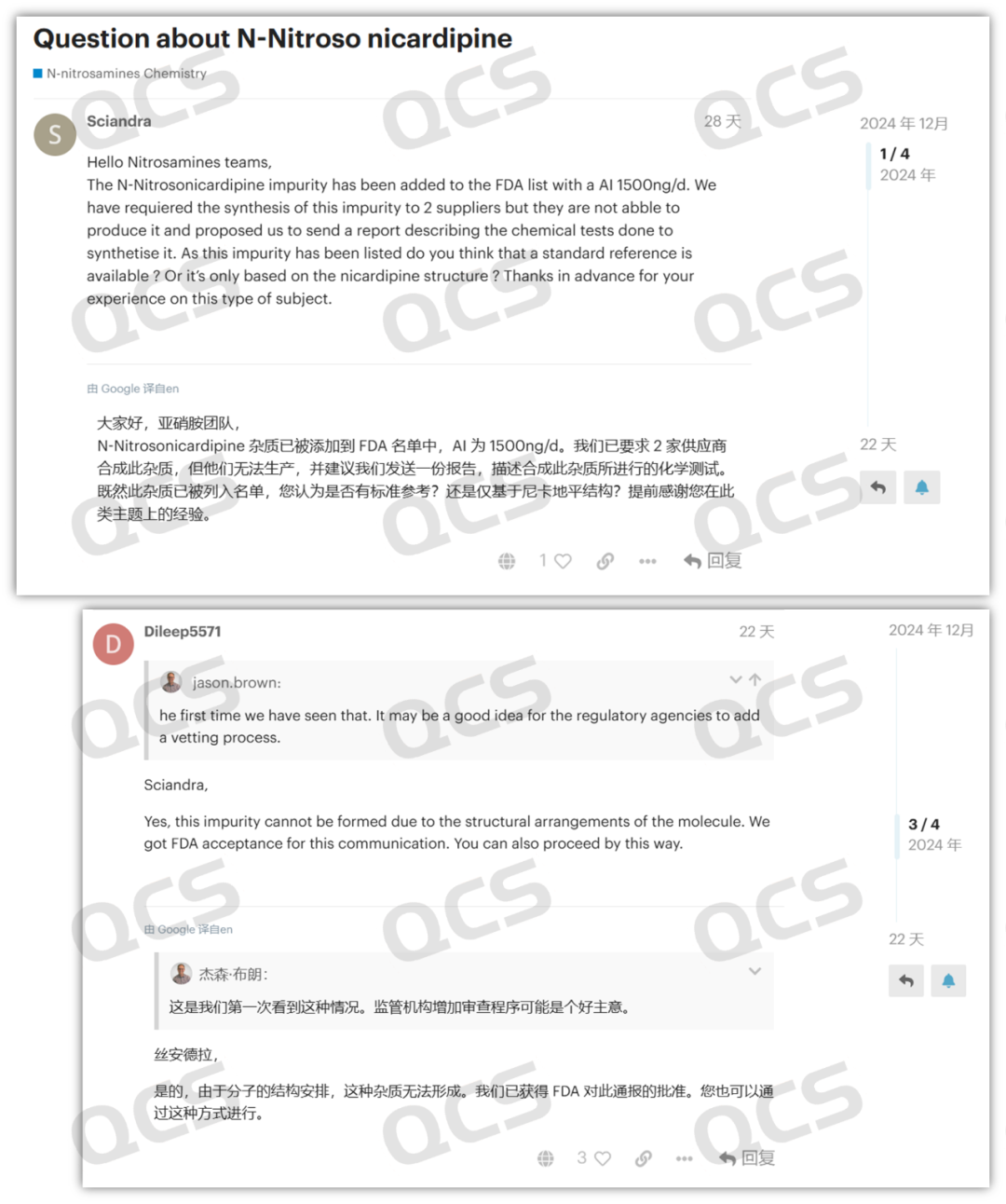
Figure 2: USP nitrosamine forum discussion screenshot 1
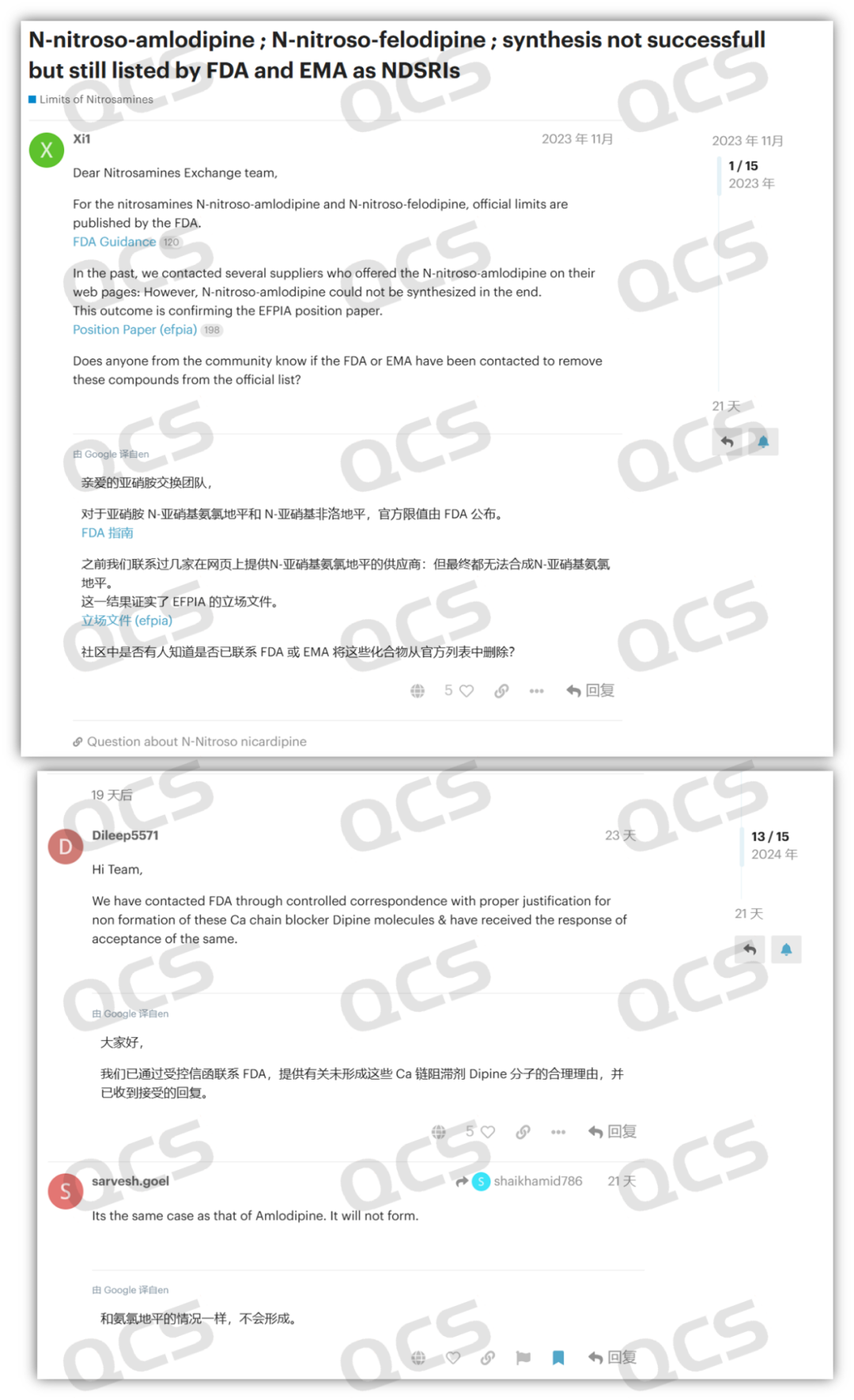
Figure 3: USP nitrosamine forum discussion screenshot 2
The above information is collected by QCS standard Material research and development Center. If there is infringement, please contact and delete. The above information is for reference only.

Attention! It has been proven that dihydropyridine calcium channel blockers (CCBs) cannot generate the corresponding nitrosamine structural impurities, and this conclusion has been accepted by the FDA.
Recently, a person claiming to be from Glenmark Pharmaceuticals in India stated that the company has confirmed that drugs ending with "-dipine" cannot generate the corresponding nitrosamine structural impurities, and this conclusion has been accepted by the FDA.

Figure 1: Screenshot of 2024
Earlier, in the 11th issue of the QCS Reference Material R & D Center's WeChat推文 in 2024, an article titled "Issue 11, 2024 | Groundbreaking Conclusion! A Guide to Avoiding Pitfalls with N - Nitrosamine Impurities in 'Dipines'" was published. In the article, based on the experiences of multiple self - developed projects, we reached the conclusion that nitrosamine impurities in dipines do not exist. Recently, we learned that a pharmaceutical company in India has submitted a declaration regarding the nitrosamine impurities in its dipine drugs according to this method, and it has been accepted by the FDA.
Recently, in the USP Nitrosamine Forum, a user named "Dileep5571" posted replies in two discussion groups, namely "Discussion on Nitrosamine Nicardipine Impurities" and "N - Nitrosamine Impurities Listed by the FDA and EMA: N - Nitrosoamlodipine and N - Nitrosfelodipine Cannot Be Synthesized". In the replies, the user stated that their company has confirmed that the N - nitroso impurities of dipine drugs cannot be synthesized, and they have submitted a declaration based on this conclusion, which has been accepted by the FDA.
Meanwhile, other users on the forum reported that the so - called dipine N - nitrosamines provided by multiple suppliers from different countries were all proven to be mislabeled products. They were identified as aromatized impurities rather than N - nitrosamine impurities. The user information indicates that the post was from Glenmark Pharmaceuticals Limited. Founded in 1977, it is one of the leading multinational pharmaceutical companies in India.

Figure 2: USP nitrosamine forum discussion screenshot 1

Figure 3: USP nitrosamine forum discussion screenshot 2
The above information is collected by QCS standard Material research and development Center. If there is infringement, please contact and delete. The above information is for reference only.

Junte-se à nossa lista de e-mails
Inscreva-se para receber atualizações sobre novos produtos, promoções e recursos!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*Todos os produtos desta empresa são destinados apenas para pesquisa científica.

*Todos os produtos desta empresa são destinados apenas para pesquisa científica.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号