Time:2024-11-25
Today, we present the study concerning the stability of hetero-impurity Tofacibute once again, a drug employed for the treatment of rheumatoid arthritis. Previously, we have dispatched an article titled Research Share on Related Impurities of Tofaciib Citrate Tablet, a Drug for Rheumatoid Arthritis Treatment, and you are welcome to click for your review. Tofacitinib citrate, being the first inhibitor of the JAK pathway in terms of mechanism of action, is a novel oral protein tyrosine kinase inhibitor that governs specific signal transduction pathways of JAK, hindering the phosphorylation and activation of signal transduction factors and transcriptional activators (STAT).
Experimental Scheme
In this experiment, our center carried out solution stability studies on 3 specific impurities of Tofaciib by referring to the chromatographic conditions employed under "Related substances" in the import registration standard of Tofaciib Citrate Tablets (Standard No. JX20130251). The sample number and structural formula utilized are presented in FIG. 1 and FIG. 2 below.
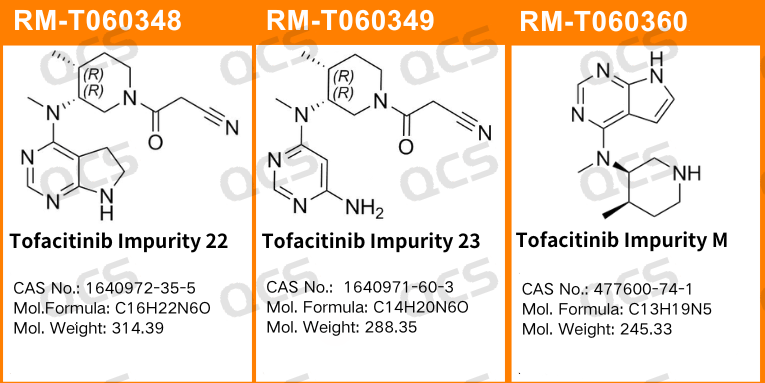
Figure 1: The number of impurity items and the structural formula employed in this study
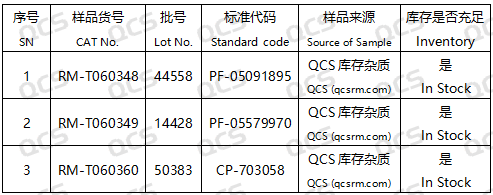
Figure 2: The corresponding relationship between the impurity code included in the standard and the number of impurity items utilized in this study
In this experiment, the experimenter utilized RM-T060348 (PF-05091895; Tofacitinib Impurity 22; CAS NO: 1640972-35-5), RM-T060349 (PF-05579970; Tofacitinib Impurity 23; CAS NO: 1640971-60-3), and RM-T060360 (CP-703058; Tofacitinib Impurity M; CAS NO: 477600-74-1). An appropriate quantity of each product was taken and placed respectively in acidic, neutral, and alkaline solutions at room temperature and pressure for 0, 3, 6, 12, and 24 hours. The chromatographic conditions were in accordance with those under "Related substances" in the import registration standard of Tofacticloth Citrate Tablets (Standard No. JX20130251). The main peak-to-peak area ratio was calculated by the area normalization method. The stability of the sample solution was determined by observing the change in the ratio of the main peak area of the sample with the extension of the sample storage time.
Experimental Conclusion
We conducted tests and found that the ratio of the main peak-peak area of sample RM-T060360 (CP-703058) showed minimal change over the 24-hour period when placed in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. As a result, it can be inferred that the sample is relatively stable when exposed to acidic, neutral, and alkaline solutions for 24 hours. The data of the main peak area of each detection point of the sample under various PH values are presented as follows:
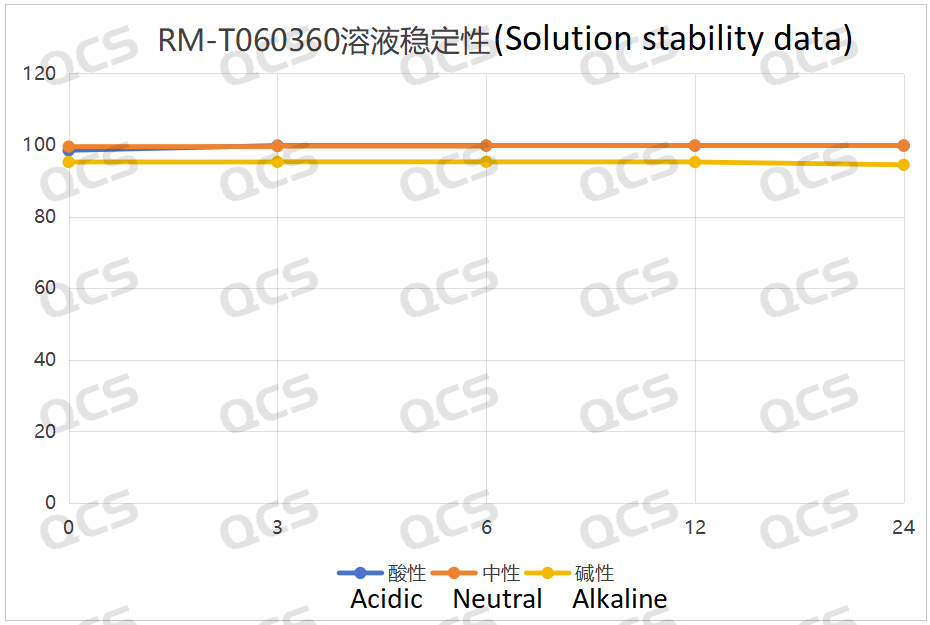
Figure 3: Line Diagram Summarizing Solution Stability Data for Sample RM-T060360 (CP-703058)
RM-T060348(PF-05091895) and RM-T060349(PF-05579970)
During the detection procedure, the experimenter discovered that the ratio of the main peak-peak area of the samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970) remained relatively stable when placed in acidic and neutral solutions for 24 hours, with the relative standard deviation being less than 2.0%. This implies that the two samples were relatively stable when exposed to acidic and neutral solutions for 24 hours. Nevertheless, when the two samples were placed in the alkaline solution for 24 hours, the proportion of the main peak area continued to decline as the placement time extended, while the proportion of the impurity peak area kept increasing. This indicates that the above two samples were unstable in the alkaline solution and would degrade over time. The main peak area data of the samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970) at each detection point under various PH values are as follows:
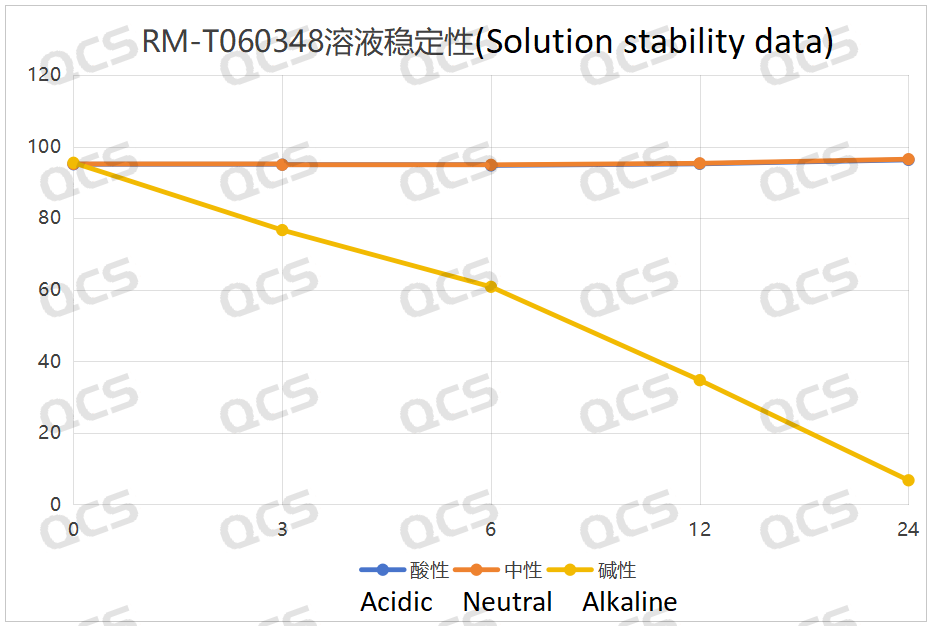
Figure 4: Line Diagram Summarizing Solution Stability Data for Sample RM-T060348 (PF-05091895)
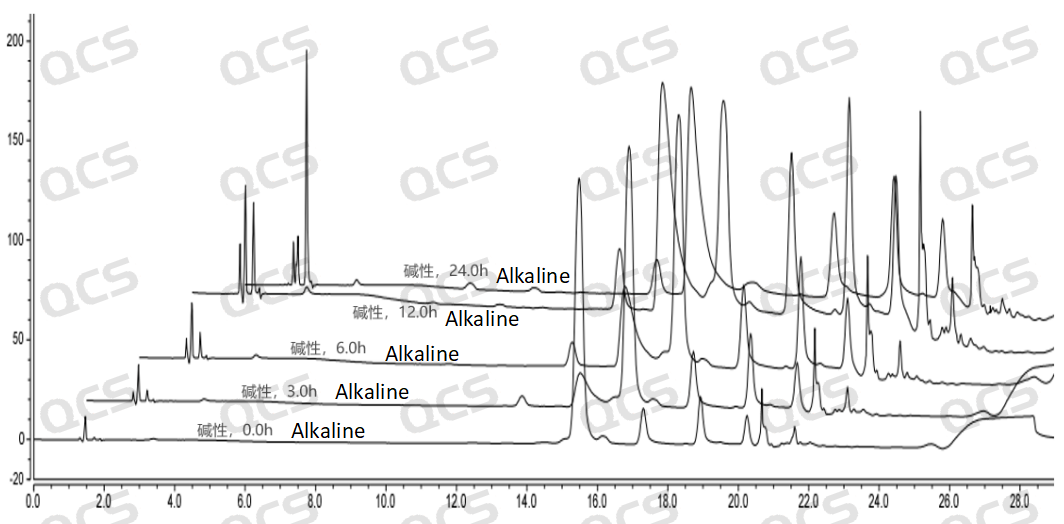
Figure 5: 3D Diagram Illustrating the Stability Data of Sample RM-T060348 (PF-05091895) after Being Placed in Alkaline Solution for Different Durations
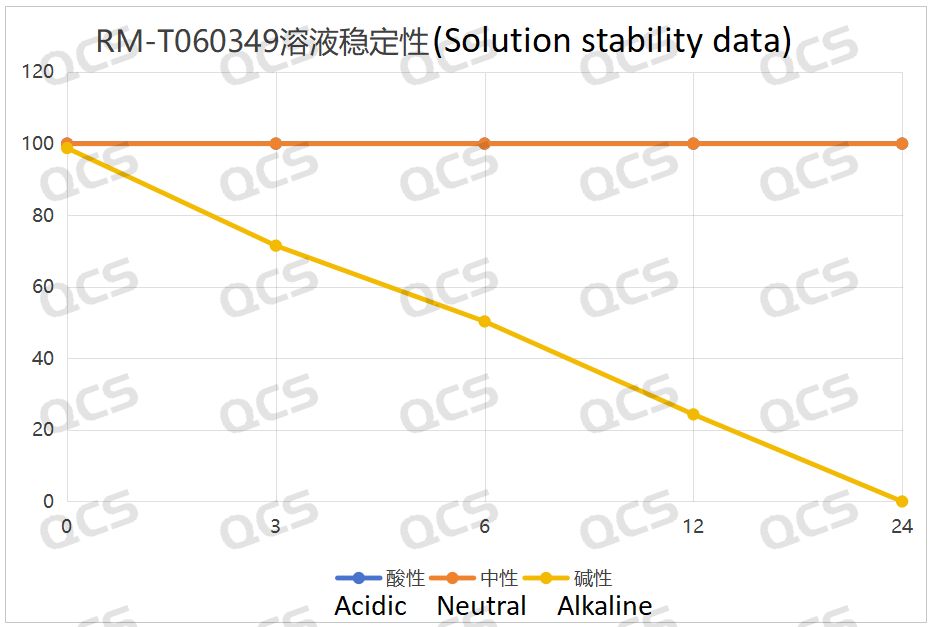
Figure 6: Line Graph Summarizing the Solution Stability Data of Sample RM-T060349 (PF-05579970)
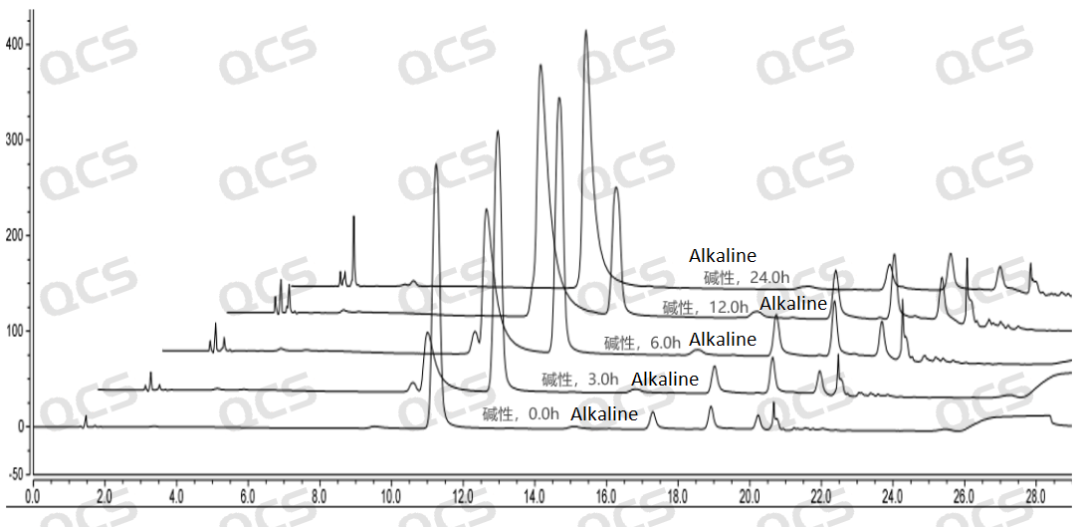
Figure 7: 3D Diagram Displaying the Stability Data of Sample RM-T060349 (PF-05579970) after Being Positioned in Alkaline Solution for Various Time Periods
Summary
In conclusion, we discovered that sample RM-T060360 (CP-703058) exhibited good stability in acidic through this experiment, neutral, and alkaline solutions. Samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970) were stable in acidic and neutral solutions, but they were less stable in alkaline solutions and would degrade over time. Therefore, customers should refrain from using alkaline diluents when testing samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970), and should not store the samples with alkaline ones for an extended period. If the customer would like to know the stability content of these three samples, we kindly invite you to reach out to our company for assistance.

Today, we present the study concerning the stability of hetero-impurity Tofacibute once again, a drug employed for the treatment of rheumatoid arthritis. Previously, we have dispatched an article titled Research Share on Related Impurities of Tofaciib Citrate Tablet, a Drug for Rheumatoid Arthritis Treatment, and you are welcome to click for your review. Tofacitinib citrate, being the first inhibitor of the JAK pathway in terms of mechanism of action, is a novel oral protein tyrosine kinase inhibitor that governs specific signal transduction pathways of JAK, hindering the phosphorylation and activation of signal transduction factors and transcriptional activators (STAT).
Experimental Scheme
In this experiment, our center carried out solution stability studies on 3 specific impurities of Tofaciib by referring to the chromatographic conditions employed under "Related substances" in the import registration standard of Tofaciib Citrate Tablets (Standard No. JX20130251). The sample number and structural formula utilized are presented in FIG. 1 and FIG. 2 below.

Figure 1: The number of impurity items and the structural formula employed in this study

Figure 2: The corresponding relationship between the impurity code included in the standard and the number of impurity items utilized in this study
In this experiment, the experimenter utilized RM-T060348 (PF-05091895; Tofacitinib Impurity 22; CAS NO: 1640972-35-5), RM-T060349 (PF-05579970; Tofacitinib Impurity 23; CAS NO: 1640971-60-3), and RM-T060360 (CP-703058; Tofacitinib Impurity M; CAS NO: 477600-74-1). An appropriate quantity of each product was taken and placed respectively in acidic, neutral, and alkaline solutions at room temperature and pressure for 0, 3, 6, 12, and 24 hours. The chromatographic conditions were in accordance with those under "Related substances" in the import registration standard of Tofacticloth Citrate Tablets (Standard No. JX20130251). The main peak-to-peak area ratio was calculated by the area normalization method. The stability of the sample solution was determined by observing the change in the ratio of the main peak area of the sample with the extension of the sample storage time.
Experimental Conclusion
We conducted tests and found that the ratio of the main peak-peak area of sample RM-T060360 (CP-703058) showed minimal change over the 24-hour period when placed in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. As a result, it can be inferred that the sample is relatively stable when exposed to acidic, neutral, and alkaline solutions for 24 hours. The data of the main peak area of each detection point of the sample under various PH values are presented as follows:

Figure 3: Line Diagram Summarizing Solution Stability Data for Sample RM-T060360 (CP-703058)
RM-T060348(PF-05091895) and RM-T060349(PF-05579970)
During the detection procedure, the experimenter discovered that the ratio of the main peak-peak area of the samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970) remained relatively stable when placed in acidic and neutral solutions for 24 hours, with the relative standard deviation being less than 2.0%. This implies that the two samples were relatively stable when exposed to acidic and neutral solutions for 24 hours. Nevertheless, when the two samples were placed in the alkaline solution for 24 hours, the proportion of the main peak area continued to decline as the placement time extended, while the proportion of the impurity peak area kept increasing. This indicates that the above two samples were unstable in the alkaline solution and would degrade over time. The main peak area data of the samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970) at each detection point under various PH values are as follows:

Figure 4: Line Diagram Summarizing Solution Stability Data for Sample RM-T060348 (PF-05091895)

Figure 5: 3D Diagram Illustrating the Stability Data of Sample RM-T060348 (PF-05091895) after Being Placed in Alkaline Solution for Different Durations

Figure 6: Line Graph Summarizing the Solution Stability Data of Sample RM-T060349 (PF-05579970)

Figure 7: 3D Diagram Displaying the Stability Data of Sample RM-T060349 (PF-05579970) after Being Positioned in Alkaline Solution for Various Time Periods
Summary
In conclusion, we discovered that sample RM-T060360 (CP-703058) exhibited good stability in acidic through this experiment, neutral, and alkaline solutions. Samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970) were stable in acidic and neutral solutions, but they were less stable in alkaline solutions and would degrade over time. Therefore, customers should refrain from using alkaline diluents when testing samples RM-T060348 (PF-05091895) and RM-T060349 (PF-05579970), and should not store the samples with alkaline ones for an extended period. If the customer would like to know the stability content of these three samples, we kindly invite you to reach out to our company for assistance.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号