Time:2024-11-05
Today, we will share the stability study of antitumor drug-Ibrutinib specific impurities, a small molecule BTK (Bruton tyrosine kinase) inhibitor. Ibrutinib forms a covalent bond with the cysteine residue of the BTK active site, thereby inhibiting the enzymatic activity of BTK. BTK is a signaling molecule in the B cell antigen receptor (BCR) and cytokine receptor pathways. BTK activates pathways necessary for B cell migration, chemotaxis, and adhesion through B cell surface receptor signaling. In non clinical research results, Ibrutinib inhibited in vivo proliferation and survival of malignant B cells, as well as in vitro cell migration and substrate adhesion. Suitable for the treatment of mantle cell lymphoma patients who have received at least one previous treatment; Monotherapy is suitable for the treatment of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma.
Experimental plan
In this experiment, our center conducted a solution stability study on three specific impurities of Ibrutinib by referring to the chromatographic conditions used under the "Related Substances" check item in "Ibrutinib Capsules" (standard number JX20160135). The sample item numbers and structural formulas used are shown in Figure 1 and Figure 2:
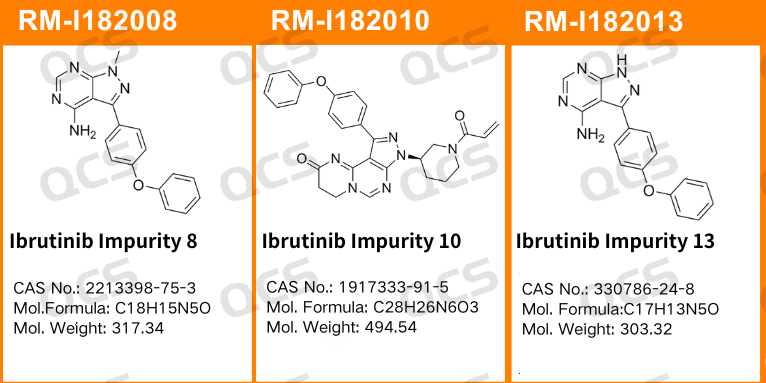
Figure 1: Impurity item numbers and structural formulas used in this study
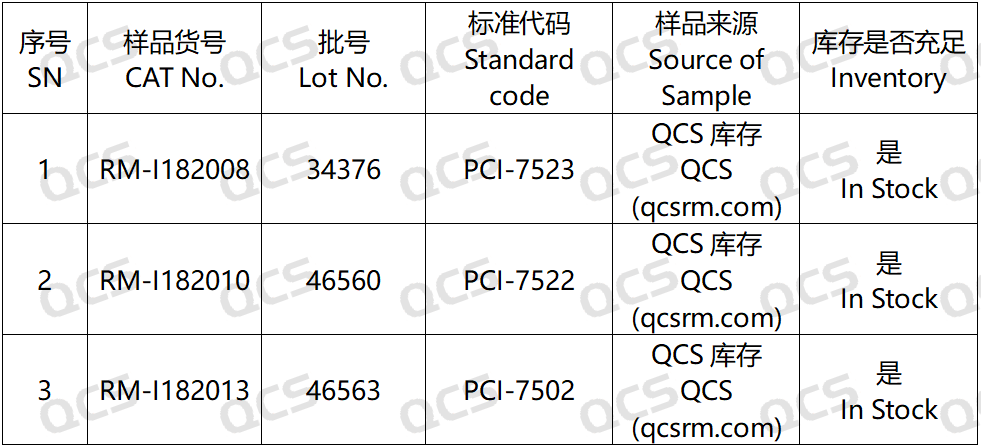
Figure 2: Correspondence between impurity codes included in the standard and impurity item numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-I182008(Ibrutinib Impurity 8; CAS NO: 2213398-75-3), RM-I182010(Ibrutinib Impurity 10; CAS NO: 1917333-91-5), and RM-I182013(Ibrutinib Impurity 13; CAS NO: 330786-24-8, then placed them in acidic, neutral, and alkaline solutions, respectively. They were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively. After that, they were injected and tested according to the chromatographic conditions used in the "Related Substances" section of "Ibrutinib Capsules" (standard number JX20160135). Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Empirical conclusion
After testing, it was found that the main peak area of sample RM-I182008 did not change significantly after being placed in acidic, neutral, and alkaline solutions for 24 hours, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-I182008 under various pH conditions are as follows
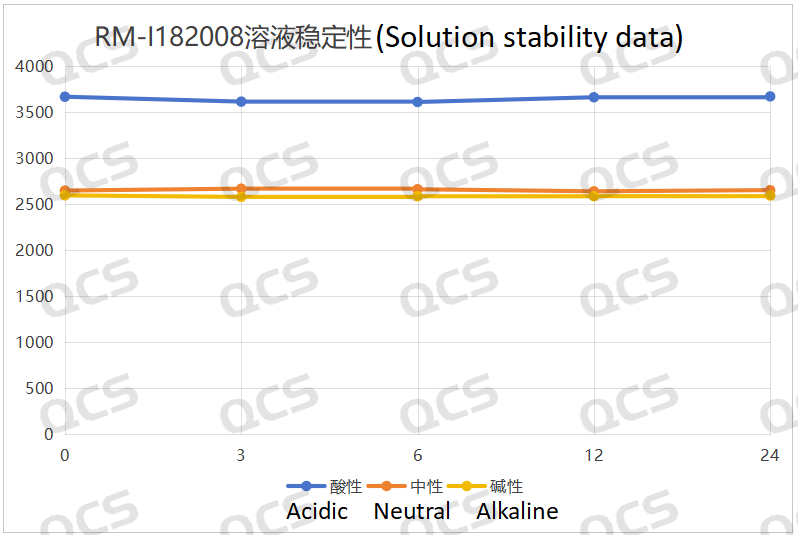
Figure 3: Summary line chart of solution stability data for sample RM-I182008
After testing, it was found that the main peak area of sample RM-I182013 did not change significantly after being placed in acidic and alkaline solutions for 24 hours, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and alkaline solutions for 24 hours The main peak area of the sample changed slightly during 24 hours in neutral solution, with a relative standard deviation of 3.99%. Moreover, comparing the sampling and testing results of the sample after being placed in neutral solution for 0, 3, 6, 12, and 24 hours, it was found that the main peak area decreased by 8.48% in the detection spectrum at the 3-hour sampling point. However, in the detection spectrum at the 6, 12, and 24 hour sampling points, the main peak area did not change and no other impurity peaks appeared. This is because the sample will partially precipitate after being dissolved and left in neutral solution for a period of time. So the sample is relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-I182013 under various pH conditions are as follows:
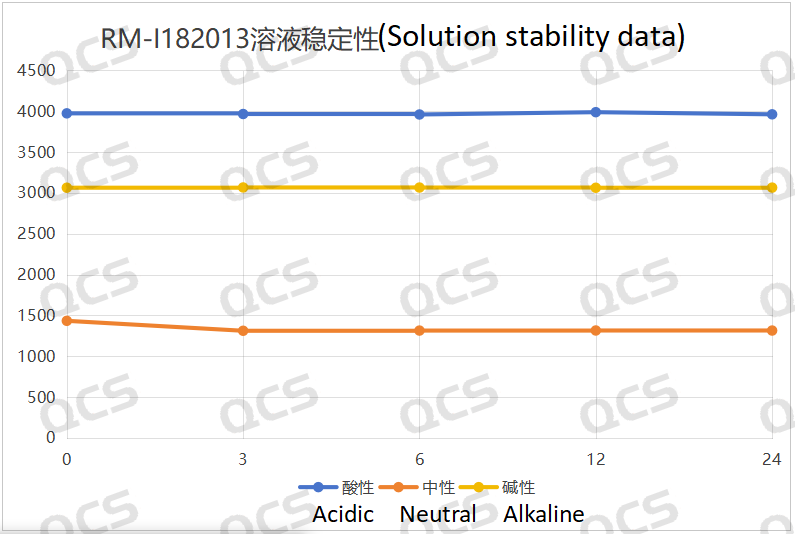
Figure 4: Summary line chart of solution stability data for sample RM-I182013
After testing, it was found that the main peak area of sample RM-I182010 changed significantly after being placed in an acidic solution for 24 hours, with a relative standard deviation greater than 2.0%. So it can be considered that the sample is unstable when left in an acidic solution for 24 hours. From the test results, it can be seen that the sample has already undergone degradation during the dissolution process with acidic diluent, and the amount of degradation of the sample increases with the extension of storage time. The detection spectrum after being left for 6 hours shows that sample RM-I182010 has undergone secondary degradation, while the detection spectrum after being left for 12 hours shows that sample RM-I182010 has completely degraded.
During the process of placing sample RM-I182010 in neutral solution for 24 hours, the main peak area did not change significantly, and the relative standard deviation was less than 2.0%. However, from the test results, it can be seen that the sample has partially degraded during the dissolution process with neutral diluent, but with the extension of storage time, the sample no longer undergoes degradation. So it can be considered that sample RM-I182010 is unstable when left in neutral solution for 24 hours.
During the 24-hour storage of sample RM-I182010 in alkaline solution, there was a significant change in the peak area of the main peak, with a relative standard deviation greater than 2.0%. So it can be considered that the sample is unstable when left in an alkaline solution for 24 hours. From the test results, it can be seen that the sample has already undergone degradation during the dissolution process with alkaline diluent, and the amount of degradation of the sample increases with the extension of storage time. The detection spectrum left for 24 hours shows that sample RM-I182010 has undergone secondary degradation.
The peak area data of the main peak at each detection point of sample RM-I182010 under various pH conditions are as follows:
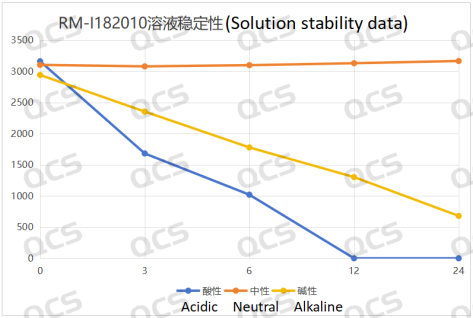
Figure 5: Summary line chart of solution stability data for sample RM-I182010
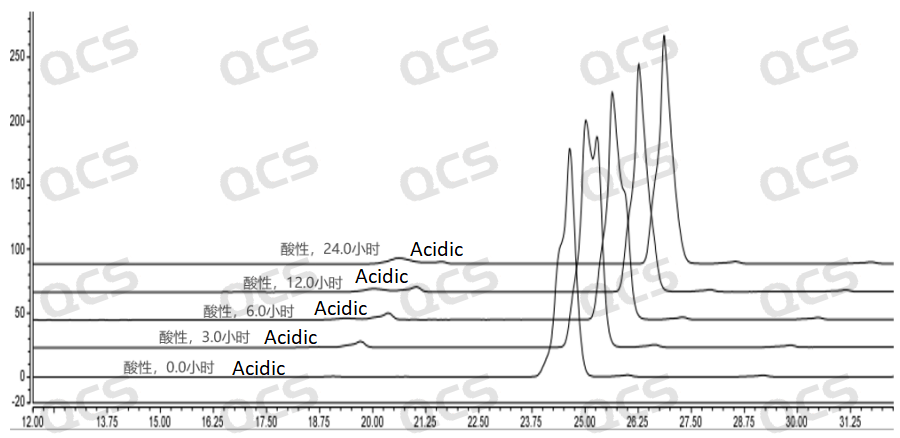
Figure 6: 3D Summary of acidic solution stability data for sample RM-I182010
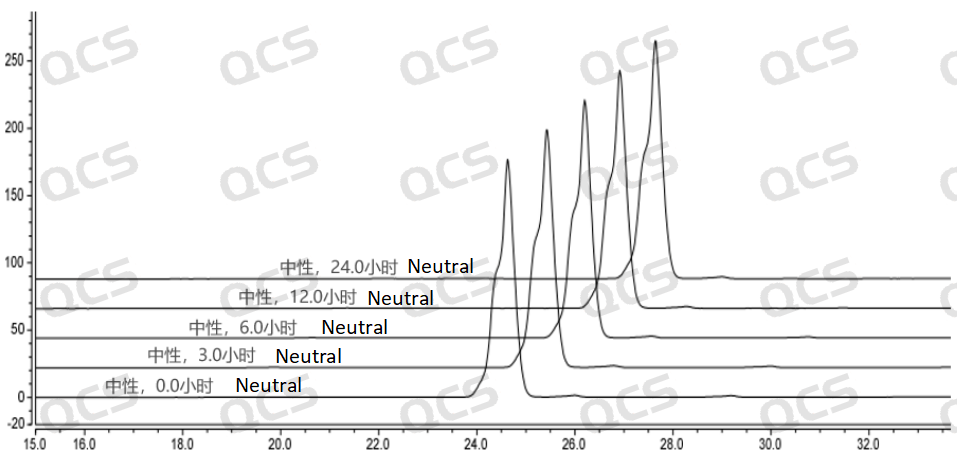
Figure 7: 3D Summary of neutral solution stability data for sample RM-I182010
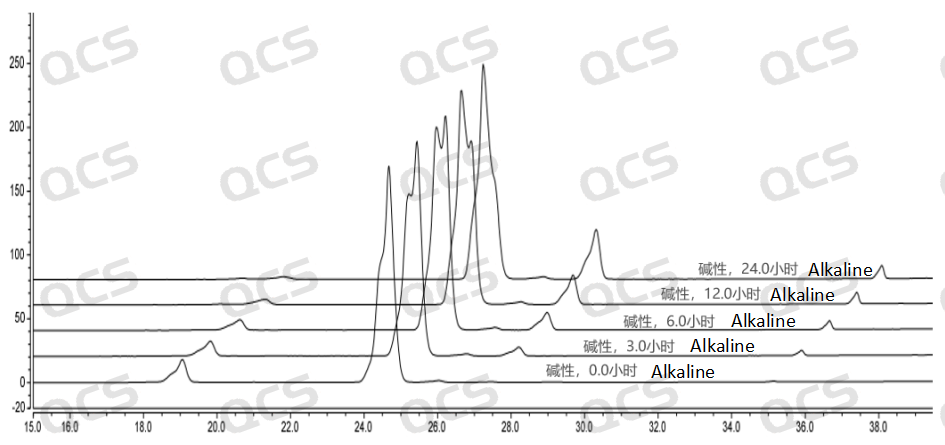
Figure 8: 3D Summary of alkaline solution stability data for sample RM-I182010
Summary
In summary, through this experiment, we found that samples RM-I182008 and RM-I182013 have good stability in acidic, neutral, and alkaline solutions. However, sample RM-I182010 is unstable in acidic, neutral, and alkaline solutions, especially in acidic and alkaline solutions where complete degradation occurs quickly, and in alkaline solutions where partial degradation occurs. Therefore, customers should not touch acid or alkali when using, transporting, and storing sample RM-I182010, and should perform on-site testing. If customers have a need for the stability content of these 3 samples, welcome to consult our company.

Today, we will share the stability study of antitumor drug-Ibrutinib specific impurities, a small molecule BTK (Bruton tyrosine kinase) inhibitor. Ibrutinib forms a covalent bond with the cysteine residue of the BTK active site, thereby inhibiting the enzymatic activity of BTK. BTK is a signaling molecule in the B cell antigen receptor (BCR) and cytokine receptor pathways. BTK activates pathways necessary for B cell migration, chemotaxis, and adhesion through B cell surface receptor signaling. In non clinical research results, Ibrutinib inhibited in vivo proliferation and survival of malignant B cells, as well as in vitro cell migration and substrate adhesion. Suitable for the treatment of mantle cell lymphoma patients who have received at least one previous treatment; Monotherapy is suitable for the treatment of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma.
Experimental plan
In this experiment, our center conducted a solution stability study on three specific impurities of Ibrutinib by referring to the chromatographic conditions used under the "Related Substances" check item in "Ibrutinib Capsules" (standard number JX20160135). The sample item numbers and structural formulas used are shown in Figure 1 and Figure 2:

Figure 1: Impurity item numbers and structural formulas used in this study

Figure 2: Correspondence between impurity codes included in the standard and impurity item numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-I182008(Ibrutinib Impurity 8; CAS NO: 2213398-75-3), RM-I182010(Ibrutinib Impurity 10; CAS NO: 1917333-91-5), and RM-I182013(Ibrutinib Impurity 13; CAS NO: 330786-24-8, then placed them in acidic, neutral, and alkaline solutions, respectively. They were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively. After that, they were injected and tested according to the chromatographic conditions used in the "Related Substances" section of "Ibrutinib Capsules" (standard number JX20160135). Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Empirical conclusion
After testing, it was found that the main peak area of sample RM-I182008 did not change significantly after being placed in acidic, neutral, and alkaline solutions for 24 hours, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-I182008 under various pH conditions are as follows

Figure 3: Summary line chart of solution stability data for sample RM-I182008
After testing, it was found that the main peak area of sample RM-I182013 did not change significantly after being placed in acidic and alkaline solutions for 24 hours, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and alkaline solutions for 24 hours The main peak area of the sample changed slightly during 24 hours in neutral solution, with a relative standard deviation of 3.99%. Moreover, comparing the sampling and testing results of the sample after being placed in neutral solution for 0, 3, 6, 12, and 24 hours, it was found that the main peak area decreased by 8.48% in the detection spectrum at the 3-hour sampling point. However, in the detection spectrum at the 6, 12, and 24 hour sampling points, the main peak area did not change and no other impurity peaks appeared. This is because the sample will partially precipitate after being dissolved and left in neutral solution for a period of time. So the sample is relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-I182013 under various pH conditions are as follows:

Figure 4: Summary line chart of solution stability data for sample RM-I182013
After testing, it was found that the main peak area of sample RM-I182010 changed significantly after being placed in an acidic solution for 24 hours, with a relative standard deviation greater than 2.0%. So it can be considered that the sample is unstable when left in an acidic solution for 24 hours. From the test results, it can be seen that the sample has already undergone degradation during the dissolution process with acidic diluent, and the amount of degradation of the sample increases with the extension of storage time. The detection spectrum after being left for 6 hours shows that sample RM-I182010 has undergone secondary degradation, while the detection spectrum after being left for 12 hours shows that sample RM-I182010 has completely degraded.
During the process of placing sample RM-I182010 in neutral solution for 24 hours, the main peak area did not change significantly, and the relative standard deviation was less than 2.0%. However, from the test results, it can be seen that the sample has partially degraded during the dissolution process with neutral diluent, but with the extension of storage time, the sample no longer undergoes degradation. So it can be considered that sample RM-I182010 is unstable when left in neutral solution for 24 hours.
During the 24-hour storage of sample RM-I182010 in alkaline solution, there was a significant change in the peak area of the main peak, with a relative standard deviation greater than 2.0%. So it can be considered that the sample is unstable when left in an alkaline solution for 24 hours. From the test results, it can be seen that the sample has already undergone degradation during the dissolution process with alkaline diluent, and the amount of degradation of the sample increases with the extension of storage time. The detection spectrum left for 24 hours shows that sample RM-I182010 has undergone secondary degradation.
The peak area data of the main peak at each detection point of sample RM-I182010 under various pH conditions are as follows:

Figure 5: Summary line chart of solution stability data for sample RM-I182010

Figure 6: 3D Summary of acidic solution stability data for sample RM-I182010

Figure 7: 3D Summary of neutral solution stability data for sample RM-I182010

Figure 8: 3D Summary of alkaline solution stability data for sample RM-I182010
Summary
In summary, through this experiment, we found that samples RM-I182008 and RM-I182013 have good stability in acidic, neutral, and alkaline solutions. However, sample RM-I182010 is unstable in acidic, neutral, and alkaline solutions, especially in acidic and alkaline solutions where complete degradation occurs quickly, and in alkaline solutions where partial degradation occurs. Therefore, customers should not touch acid or alkali when using, transporting, and storing sample RM-I182010, and should perform on-site testing. If customers have a need for the stability content of these 3 samples, welcome to consult our company.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号