Time:2024-11-08
Today, we present the stability study of ibuprofen-specific impurities in NSAIDs. Ibuprofen is classified as a non-steroidal anti-inflammatory drug (NSAID) primarily utilized for alleviating mild to moderate pain, including headaches, toothaches, muscle aches, and joint discomfort. Additionally, it is frequently employed to reduce fever and mitigate inflammation.
Experiment Scheme
In this study, our center conducted an investigation into the stability of solutions containing three specific impurities of ibuprofen, adhering to the chromatographic conditions outlined in the 'Related Substances' section of the European Pharmacopoeia, edition 11.0. The sample number and structural formulas utilized are presented in Figures 1 and 2 below.
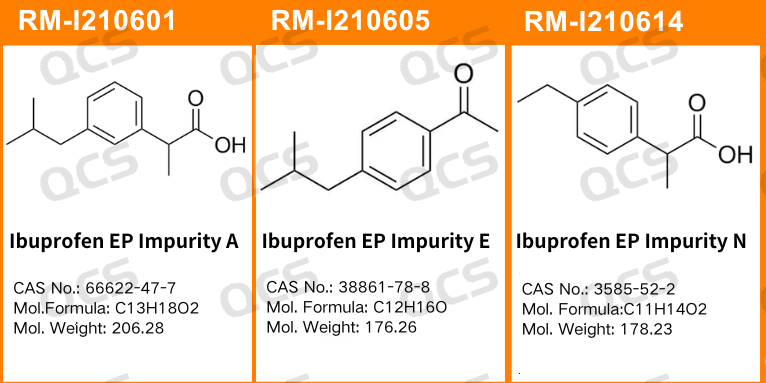
Figure 1: The impurity item numbers and structural formulas utilized in this study
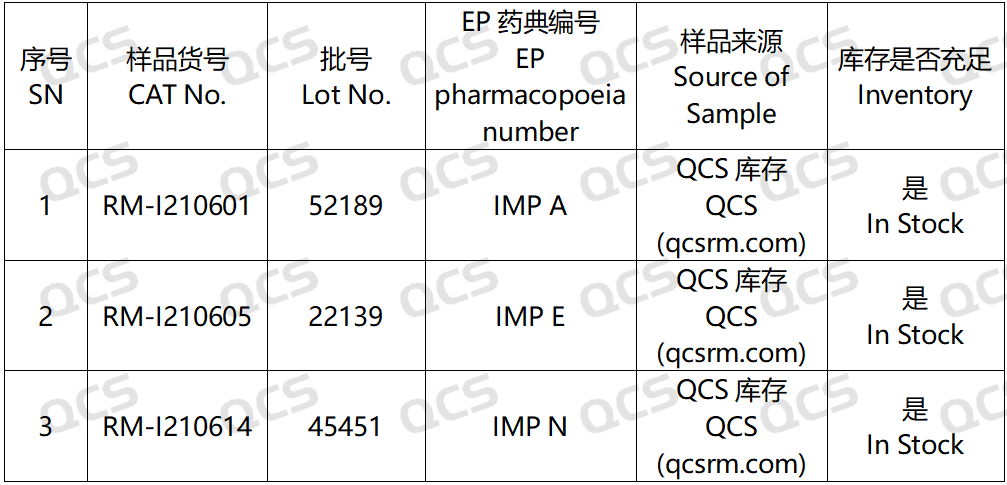
Figure 2: The correlation between the impurity codes specified in the standard and the impurity item numbers employed in this study
In this experiment, researchers employed RM-I210601 (Ibuprofen EP Impurity A; CAS NO: 66622-47-7), RM-I210605 (Ibuprofen EP Impurity E; CAS NO: 38861-78-8), and RM-I210614 (Ibuprofen EP Impurity N; CAS NO: 3585-52-2). An appropriate quantity of each sample was extracted and subsequently placed in acidic, neutral, and alkaline solutions at room temperature and atmospheric pressure for durations of 0, 3, 6, 12, and 24 hours. This procedure adhered to the chromatographic conditions specified under the section "Related substances" for "IBUPROFEN," as outlined in edition 11.0 of the European Pharmacopoeia. The stability of the sample solution was assessed by monitoring changes in the main peak area within the chromatogram over time.
Experiment Conclusion
Upon conducting tests, it was observed that the peak-to-peak area of samples RM-I210601, RM-I210605, and RM-I210614 exhibited minimal variation over a 24-hour period when immersed in acidic, neutral, and alkaline solutions, with a relative standard deviation remaining below 2.0%. Consequently, it can be inferred that these three samples demonstrate relative stability when subjected to such conditions for 24 hours. The main peak area data for each detection point of the aforementioned samples across different pH values is presented as follows:
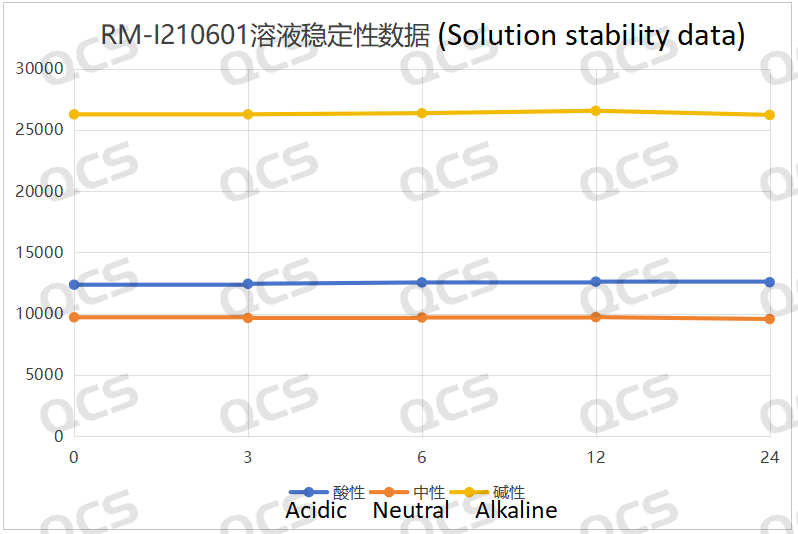
Figure 3: Line diagram summarizing the solution stability data for sample RM-I210601
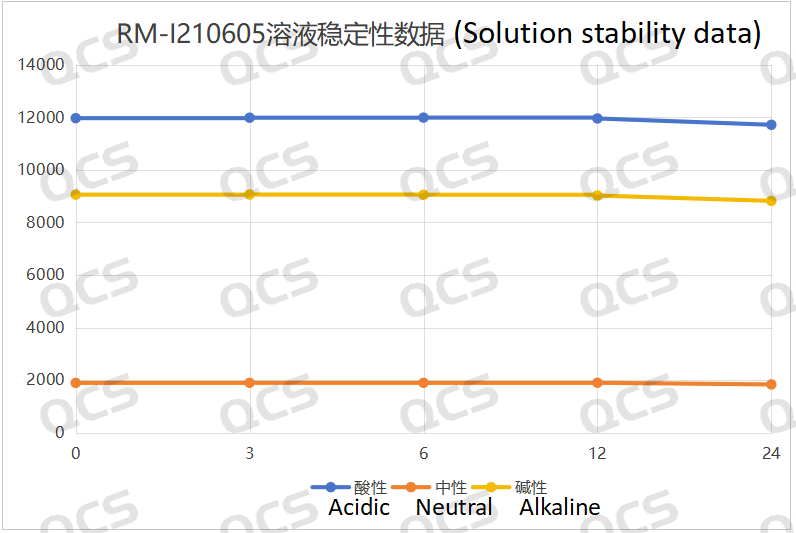
Figure 4: Line diagram summarizing the solution stability data for sample RM-I210605
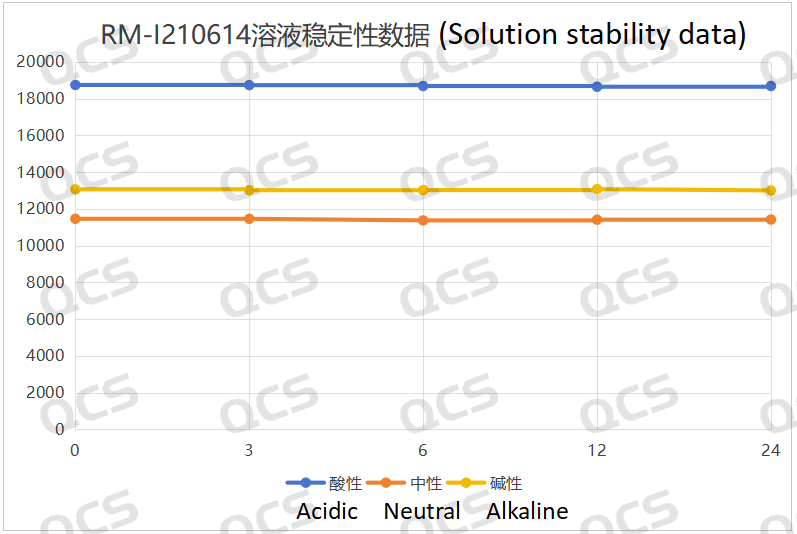
Figure 5: Line diagram summarizing the solution stability data for sample RM-I210614
Summary
To sum up, our experiment showed that samples RM-I210601, RM-I210605, and RM-I210614 performed well in terms of stability across acidic, alkaline, and neutral solutions. If you would like more information about the stability of these three samples, please feel free to reach out to us.

Today, we present the stability study of ibuprofen-specific impurities in NSAIDs. Ibuprofen is classified as a non-steroidal anti-inflammatory drug (NSAID) primarily utilized for alleviating mild to moderate pain, including headaches, toothaches, muscle aches, and joint discomfort. Additionally, it is frequently employed to reduce fever and mitigate inflammation.
Experiment Scheme
In this study, our center conducted an investigation into the stability of solutions containing three specific impurities of ibuprofen, adhering to the chromatographic conditions outlined in the 'Related Substances' section of the European Pharmacopoeia, edition 11.0. The sample number and structural formulas utilized are presented in Figures 1 and 2 below.

Figure 1: The impurity item numbers and structural formulas utilized in this study

Figure 2: The correlation between the impurity codes specified in the standard and the impurity item numbers employed in this study
In this experiment, researchers employed RM-I210601 (Ibuprofen EP Impurity A; CAS NO: 66622-47-7), RM-I210605 (Ibuprofen EP Impurity E; CAS NO: 38861-78-8), and RM-I210614 (Ibuprofen EP Impurity N; CAS NO: 3585-52-2). An appropriate quantity of each sample was extracted and subsequently placed in acidic, neutral, and alkaline solutions at room temperature and atmospheric pressure for durations of 0, 3, 6, 12, and 24 hours. This procedure adhered to the chromatographic conditions specified under the section "Related substances" for "IBUPROFEN," as outlined in edition 11.0 of the European Pharmacopoeia. The stability of the sample solution was assessed by monitoring changes in the main peak area within the chromatogram over time.
Experiment Conclusion
Upon conducting tests, it was observed that the peak-to-peak area of samples RM-I210601, RM-I210605, and RM-I210614 exhibited minimal variation over a 24-hour period when immersed in acidic, neutral, and alkaline solutions, with a relative standard deviation remaining below 2.0%. Consequently, it can be inferred that these three samples demonstrate relative stability when subjected to such conditions for 24 hours. The main peak area data for each detection point of the aforementioned samples across different pH values is presented as follows:

Figure 3: Line diagram summarizing the solution stability data for sample RM-I210601

Figure 4: Line diagram summarizing the solution stability data for sample RM-I210605

Figure 5: Line diagram summarizing the solution stability data for sample RM-I210614
Summary
To sum up, our experiment showed that samples RM-I210601, RM-I210605, and RM-I210614 performed well in terms of stability across acidic, alkaline, and neutral solutions. If you would like more information about the stability of these three samples, please feel free to reach out to us.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号