Time:2023-11-23
Introduction: Today, we are presenting a study on the impurity profile of Voronafib, a prominent product from Takeda. According to data sourced from the official website of YAOZH, there are currently over 30 research and development entities engaged in investigations related to this product. However, internal data from QCS reveals that more than 60 research institutions have conducted studies on this compound. Moreover, the number of companies that have successfully completed the evaluation process for this product has exceeded 3, highlighting the intense competition in this field.
Vonoprazan (vonoprazan, TAK-438), formerly known as Vorolanib and marketed under the trade name Volok, belongs to the P-CABs family and is a novel type of anti-acid medication. Its mechanism of action gives it characteristics such as rapid onset, strong effects, and long-lasting acid suppression.
Currently, the official website of QCS has included a total of over 75 impurities of Voronolabin (Scan the QR code at the end of this article to view the full list of impurities). Our center has conducted relevant research on the main impurities of Voronolabin according to the import registration standard for Fumaric Acid Voronolabin Tablets (Standard No. JX20190049). Some specific structural information on key impurities from customers is shown in Figure 1.
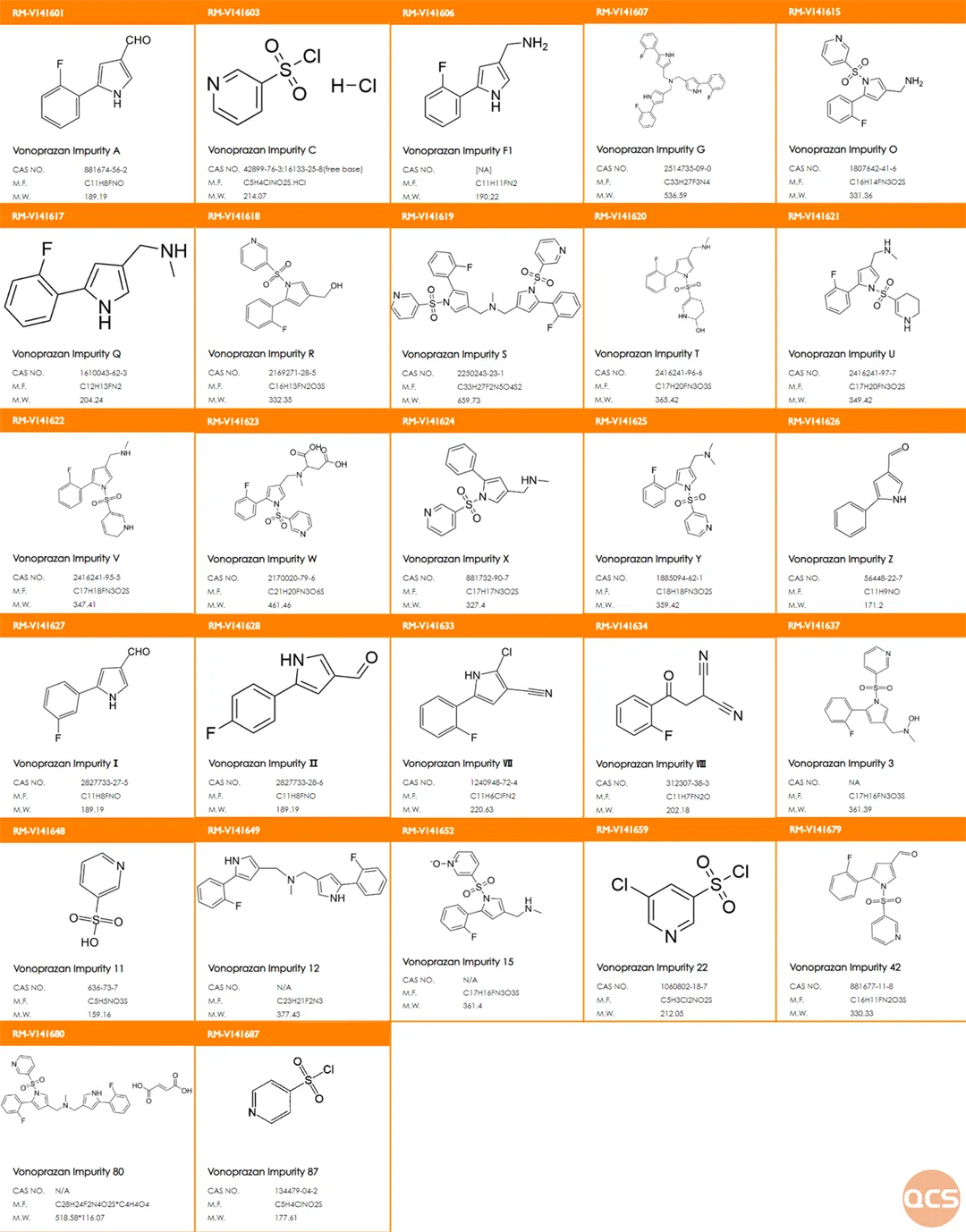
Figure 1: Vornado's customer-focused study of key impurity list
According to the impurity method specified in the import registration standard for Fumaric Acid Voronaxibin Tablets (Standard No. JX20190049), our center primarily conducted qualitative and quantitative research on the following 33 impurities. The chromatographic data for these impurities are shown in Figure 2 (the peaks labeled with signals are the impurities included in the import registration standard, while the peaks for other products are not directly labeled):
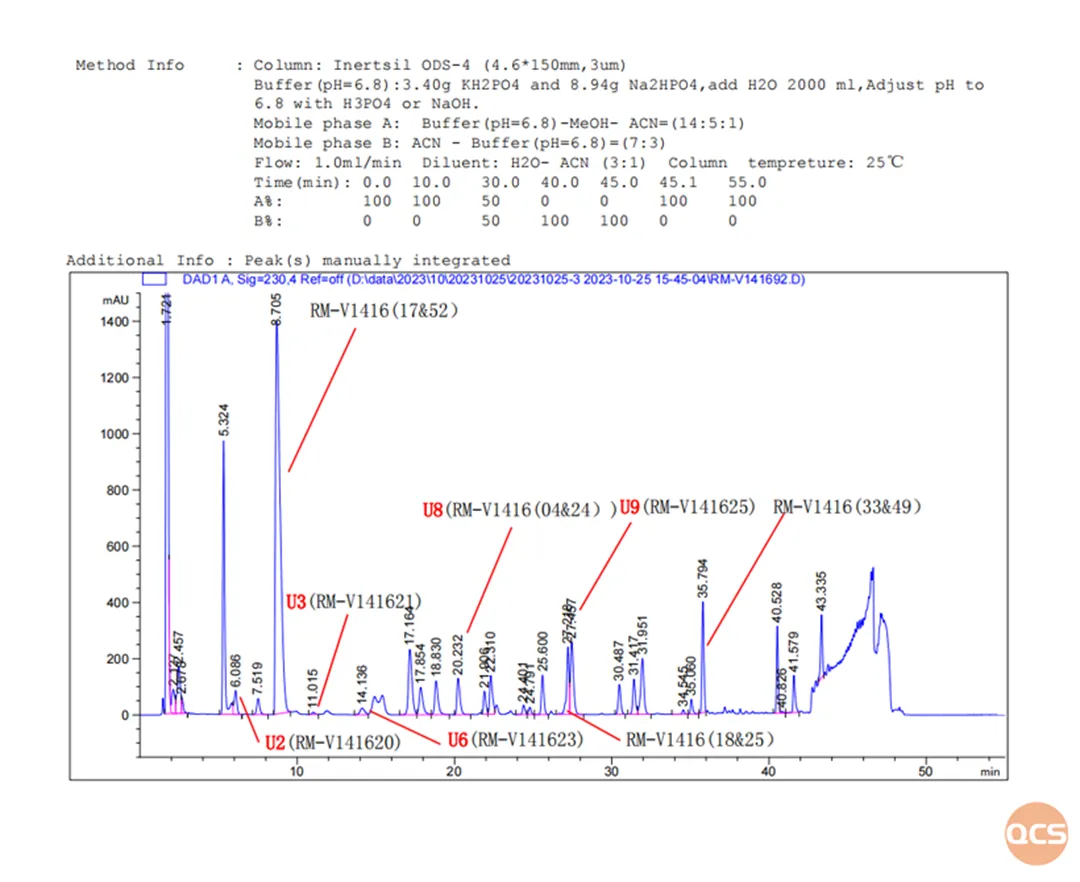
Figure 2: Chromatograms of 33 Impurities for Voronafloxacin
In this study, the QCS Research Center accessed a total of 33 impurity products from stock for a comprehensive investigation and calculated the relative retention time data for all impurity products (see Figure 4). Although the stationary phase of the chromatographic column used in this study did not exactly match the standard, the measured relative retention times were found to be largely consistent with those specified in the import registration standard for Voronafloxacin tablets, based on five code impurities. Therefore, it can be inferred that this study has essentially replicated the chromatographic results outlined in the import registration standard. Specific data are presented in Figure 3:
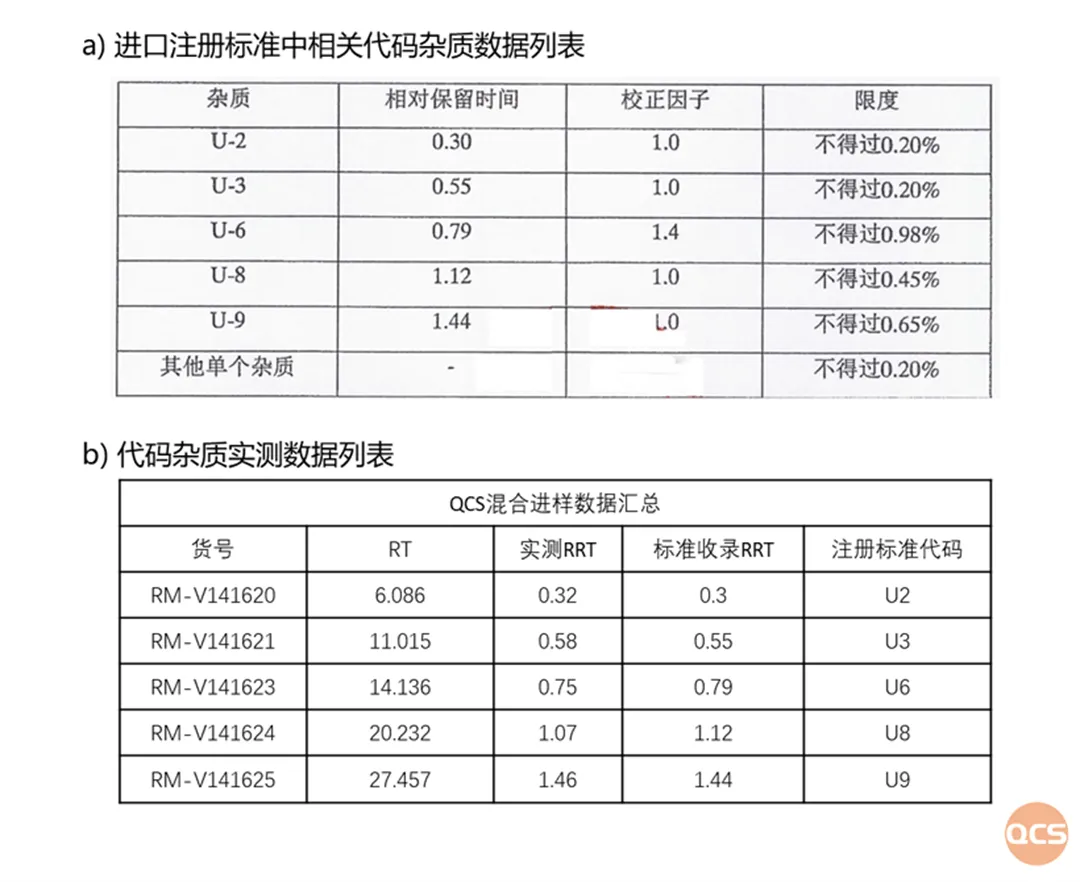
Figure 3: Impurity-related standards and verification data in the import registration standard
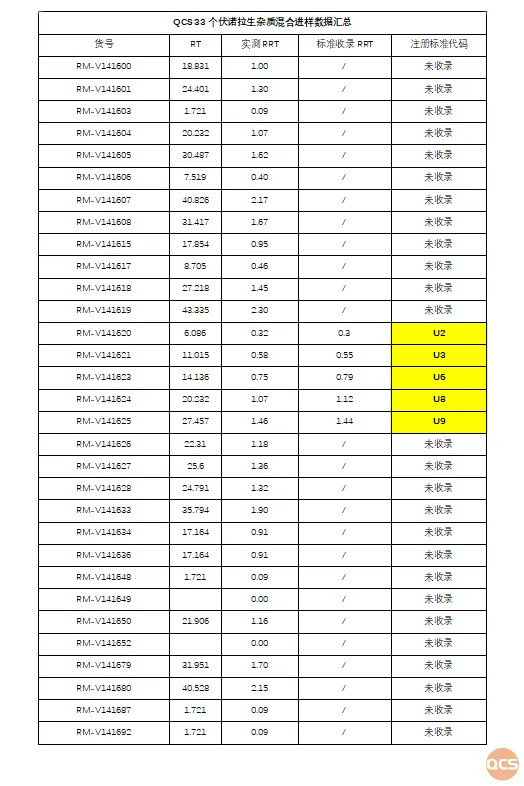
Figure 4: Summary of 33 impurity data
The aim of this study was to conduct a comparative analysis of 33 products from the QCS standard material research center under uniform chromatographic conditions. From the data presented in Figure 2, it is evident that simultaneous injection of all 33 impurities under these chromatographic conditions leads to some impurities co-eluting and thus cannot be effectively separated. For example, RM-V1416(04&24), RM-V1416(17&52), RM-V1416(18&25), and RM-V1416(33&49) could not be effectively resolved under these conditions. Therefore, when run separately under the same conditions, good separation was achieved as depicted in the specific chromatograms shown in Figure 5:
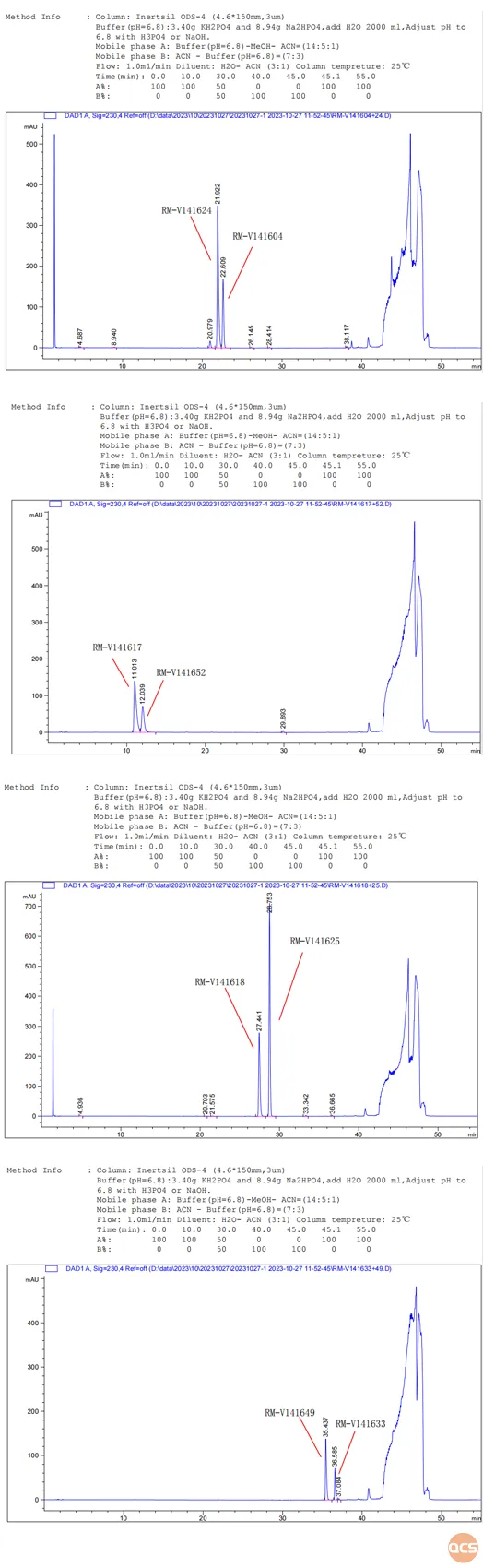
Figure 5: Four pairs of impurity mixtures that cannot be effectively separate in the mixed sample data
The chromatographic results may be affected by the injection of multiple samples into a single mixture. In order to provide more comprehensive chromatographic reference information, the QCS Standard Material Development Center conducted separate injection tests for an additional 33 selected impurity products. By combining the individual test results with those of the mixed samples, we aim to offer a more thorough reference for vornarolib impurity research. If necessary, please feel free to contact us for access to individual chromatographic results for these impurity products.

Figure 6: Overview of the Chromatographic Results for Impurity-Independent Injection of Vornorazin
Vornorazin is a well-established and widely used variety, resulting in comprehensive research on its impurities. If there are any products of particular interest that have not been addressed in this study, we welcome further discussion.
Long-press to scan the QR code and access the complete list of impurities!

The answer to the previous question is now revealed.
Question:
What are the structural formulas of the two code impurities mentioned in the registration standard, PF-06932644 and PF-06932648?
A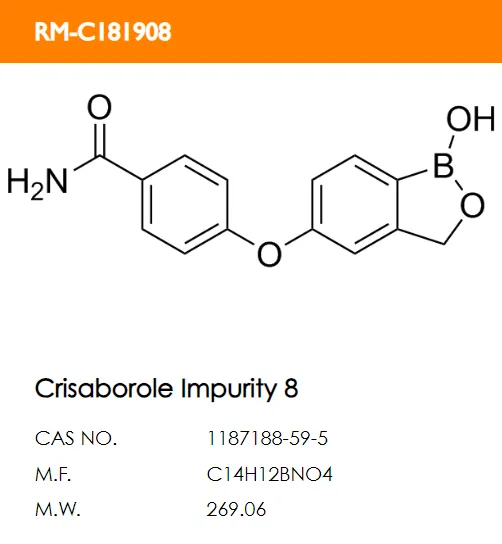 B.
B.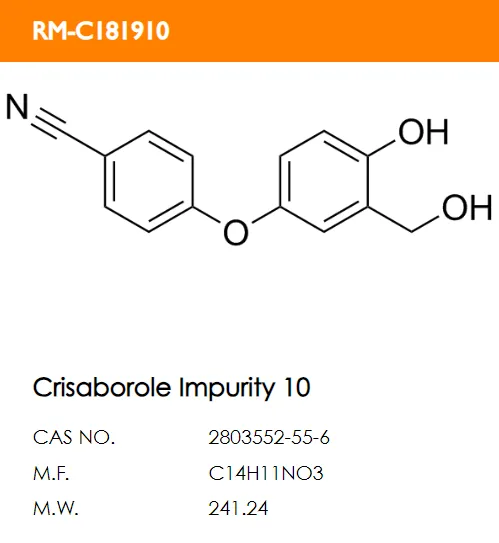 C.
C.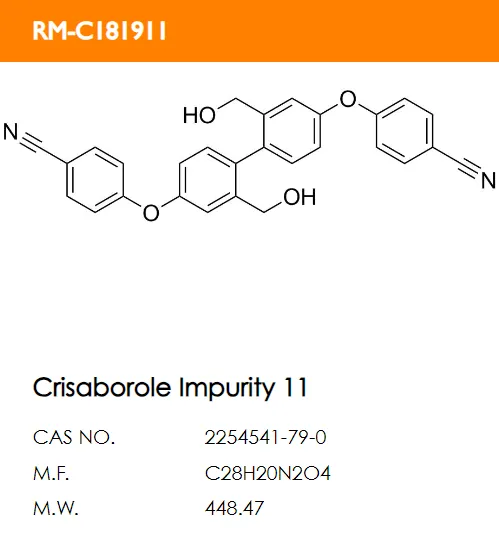
D.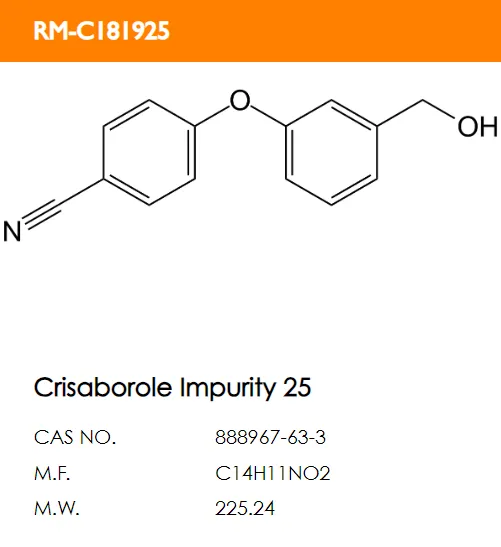 E.
E.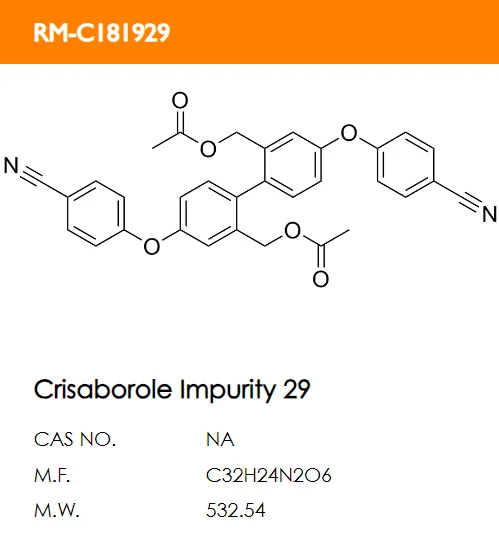 F.
F.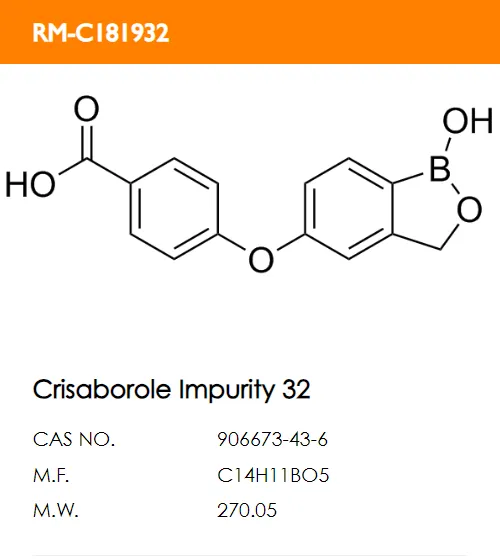
F
Correct answer: B and D.

Introduction: Today, we are presenting a study on the impurity profile of Voronafib, a prominent product from Takeda. According to data sourced from the official website of YAOZH, there are currently over 30 research and development entities engaged in investigations related to this product. However, internal data from QCS reveals that more than 60 research institutions have conducted studies on this compound. Moreover, the number of companies that have successfully completed the evaluation process for this product has exceeded 3, highlighting the intense competition in this field.
Vonoprazan (vonoprazan, TAK-438), formerly known as Vorolanib and marketed under the trade name Volok, belongs to the P-CABs family and is a novel type of anti-acid medication. Its mechanism of action gives it characteristics such as rapid onset, strong effects, and long-lasting acid suppression.
Currently, the official website of QCS has included a total of over 75 impurities of Voronolabin (Scan the QR code at the end of this article to view the full list of impurities). Our center has conducted relevant research on the main impurities of Voronolabin according to the import registration standard for Fumaric Acid Voronolabin Tablets (Standard No. JX20190049). Some specific structural information on key impurities from customers is shown in Figure 1.

Figure 1: Vornado's customer-focused study of key impurity list
According to the impurity method specified in the import registration standard for Fumaric Acid Voronaxibin Tablets (Standard No. JX20190049), our center primarily conducted qualitative and quantitative research on the following 33 impurities. The chromatographic data for these impurities are shown in Figure 2 (the peaks labeled with signals are the impurities included in the import registration standard, while the peaks for other products are not directly labeled):

Figure 2: Chromatograms of 33 Impurities for Voronafloxacin
In this study, the QCS Research Center accessed a total of 33 impurity products from stock for a comprehensive investigation and calculated the relative retention time data for all impurity products (see Figure 4). Although the stationary phase of the chromatographic column used in this study did not exactly match the standard, the measured relative retention times were found to be largely consistent with those specified in the import registration standard for Voronafloxacin tablets, based on five code impurities. Therefore, it can be inferred that this study has essentially replicated the chromatographic results outlined in the import registration standard. Specific data are presented in Figure 3:

Figure 3: Impurity-related standards and verification data in the import registration standard

Figure 4: Summary of 33 impurity data
The aim of this study was to conduct a comparative analysis of 33 products from the QCS standard material research center under uniform chromatographic conditions. From the data presented in Figure 2, it is evident that simultaneous injection of all 33 impurities under these chromatographic conditions leads to some impurities co-eluting and thus cannot be effectively separated. For example, RM-V1416(04&24), RM-V1416(17&52), RM-V1416(18&25), and RM-V1416(33&49) could not be effectively resolved under these conditions. Therefore, when run separately under the same conditions, good separation was achieved as depicted in the specific chromatograms shown in Figure 5:

Figure 5: Four pairs of impurity mixtures that cannot be effectively separate in the mixed sample data
The chromatographic results may be affected by the injection of multiple samples into a single mixture. In order to provide more comprehensive chromatographic reference information, the QCS Standard Material Development Center conducted separate injection tests for an additional 33 selected impurity products. By combining the individual test results with those of the mixed samples, we aim to offer a more thorough reference for vornarolib impurity research. If necessary, please feel free to contact us for access to individual chromatographic results for these impurity products.

Figure 6: Overview of the Chromatographic Results for Impurity-Independent Injection of Vornorazin
Vornorazin is a well-established and widely used variety, resulting in comprehensive research on its impurities. If there are any products of particular interest that have not been addressed in this study, we welcome further discussion.
Long-press to scan the QR code and access the complete list of impurities!

The answer to the previous question is now revealed.
Question:
What are the structural formulas of the two code impurities mentioned in the registration standard, PF-06932644 and PF-06932648?
A B.
B. C.
C.
D. E.
E. F.
F.
F
Correct answer: B and D.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号