Time:2023-12-01
Introduction: Today, we will discuss the impurities study of tofacitinib citrate tablets, a popular drug for rheumatoid arthritis treatment produced by Pfizer. Currently, there are 34 registration approvals for tofacitinib products and raw materials in China, including 4 approvals for finished products and 30 approvals for raw materials. Chinese companies such as ZHEJIANG HISUN PHARMACEUTICAL Co., LTD. have developed generic versions of the drug and have passed the consistency evaluation.
Tofacitinib citrate is the first JAK pathway inhibitor with a novel oral protein tyrosine kinase inhibitor mechanism, which can regulate the specific signal transduction pathway of JAK and prevent the phosphorylation and activation of signal transduction factors and transcription activators (STAT).
The official QCS website currently lists over 99 impurities of tofacitinib (Scan the QR code at the end of this article for the complete list of impurities). Our center has conducted relevant research on the primary impurities of tofacitinib, aligning with the import registration standard for Citric Acid Tofacitinib Tablets (Standard No. JX20130251). The structural information for both raw materials and specific customer-focused impurities is detailed in Figure 1.
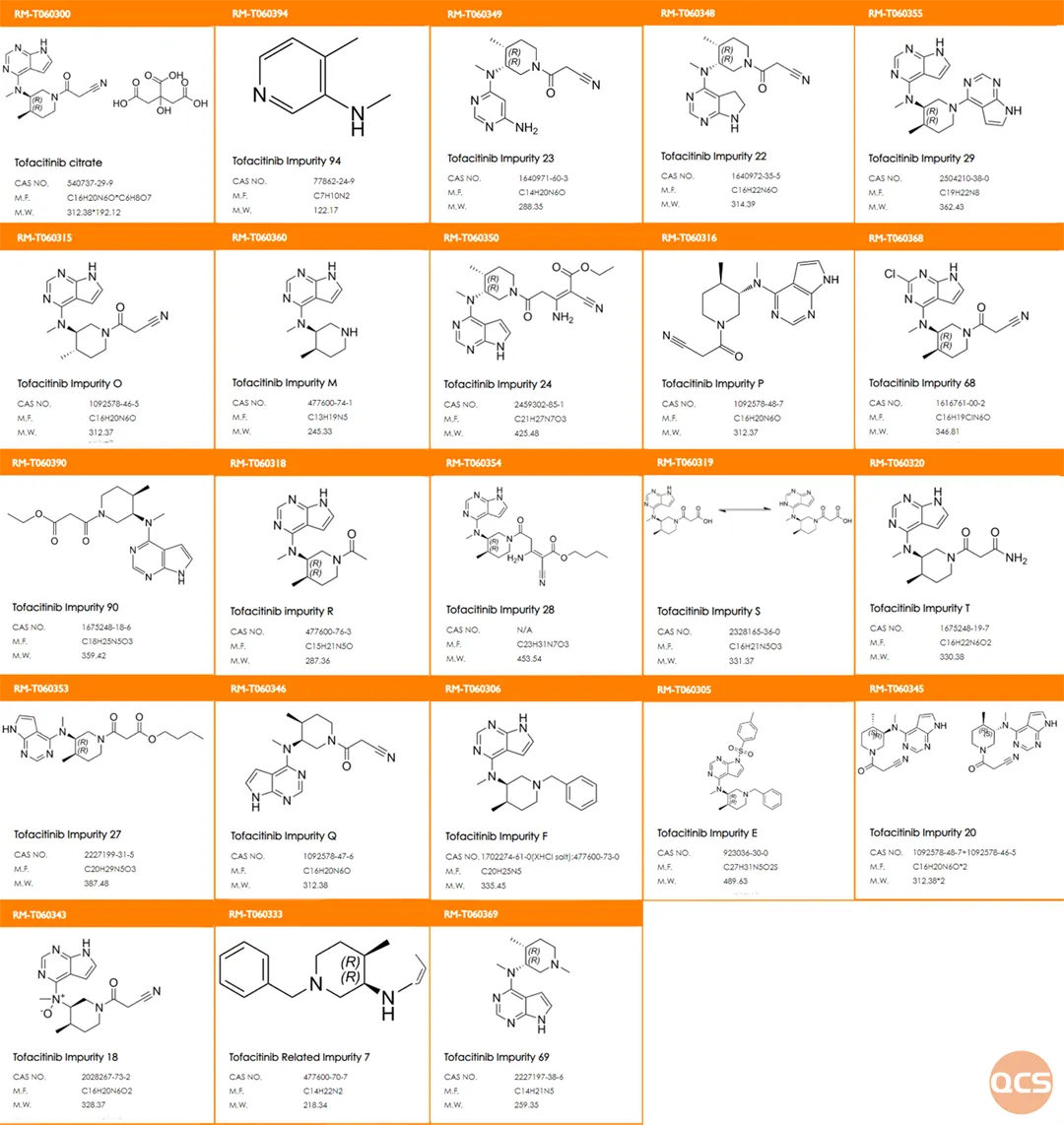
Figure 1: List of Key Impurities for Tofacitinib Customers
Based on the substance method outlined in the import registration standard for Tofacatib Citrate Tablets (Standard No. JX20130251), our center primarily conducted qualitative localization research on 22 specific impurities, with detailed chromatographic data presented in FIG. 2 and FIG. 3.
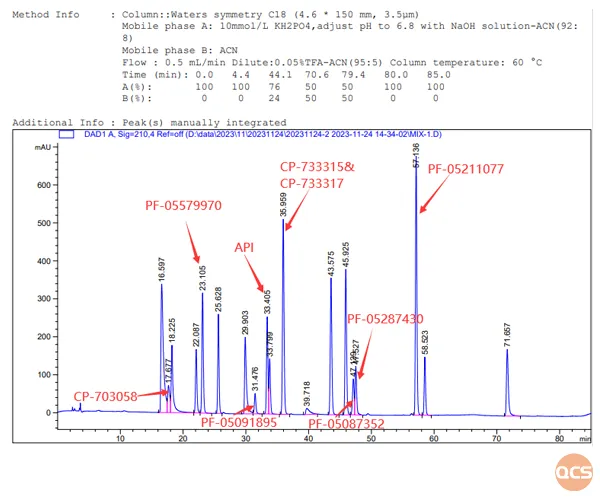
Figure 2: Location chromatogram and data summary of 22 impurities in tofacitinib
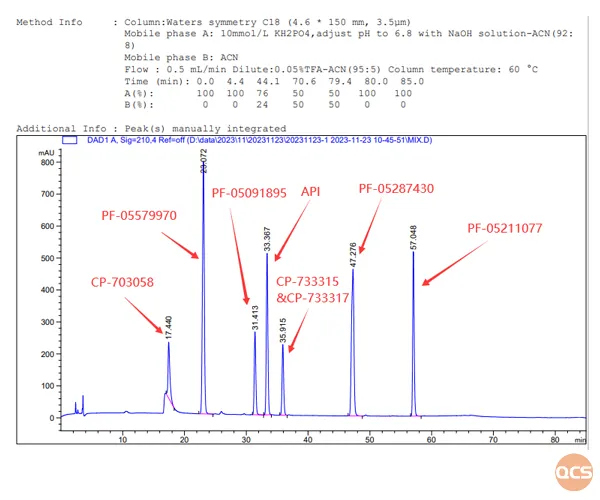
Figure 3: Location chromatogram and data summary of 8 specified impurities (excluding impurities with code PF-05198213) in the Tofacatib API and import registration standard.
In this investigation, QCS R&D Center meticulously selected 23 impurity products from its library for comprehensive analysis and determined their respective relative retention times (refer to Figure 4). Despite variations between our chromatographic column's stationary phase and that of established standards, we utilized seven impurities coded under PF-05198213 from Tofacitile citrate tablet's import registration standard as reference points. The measured relative retention times closely aligned with those specified in these standards, indicating a high degree of consistency. Detailed data is available in Figure 4.
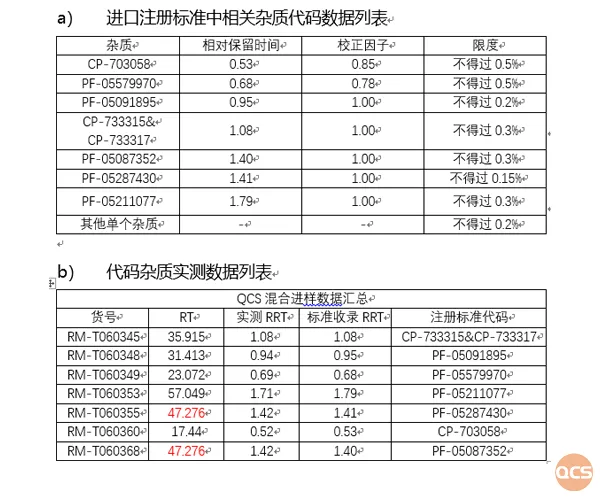
Figure 4: Code impurity-related standards and verification data in import registration standards
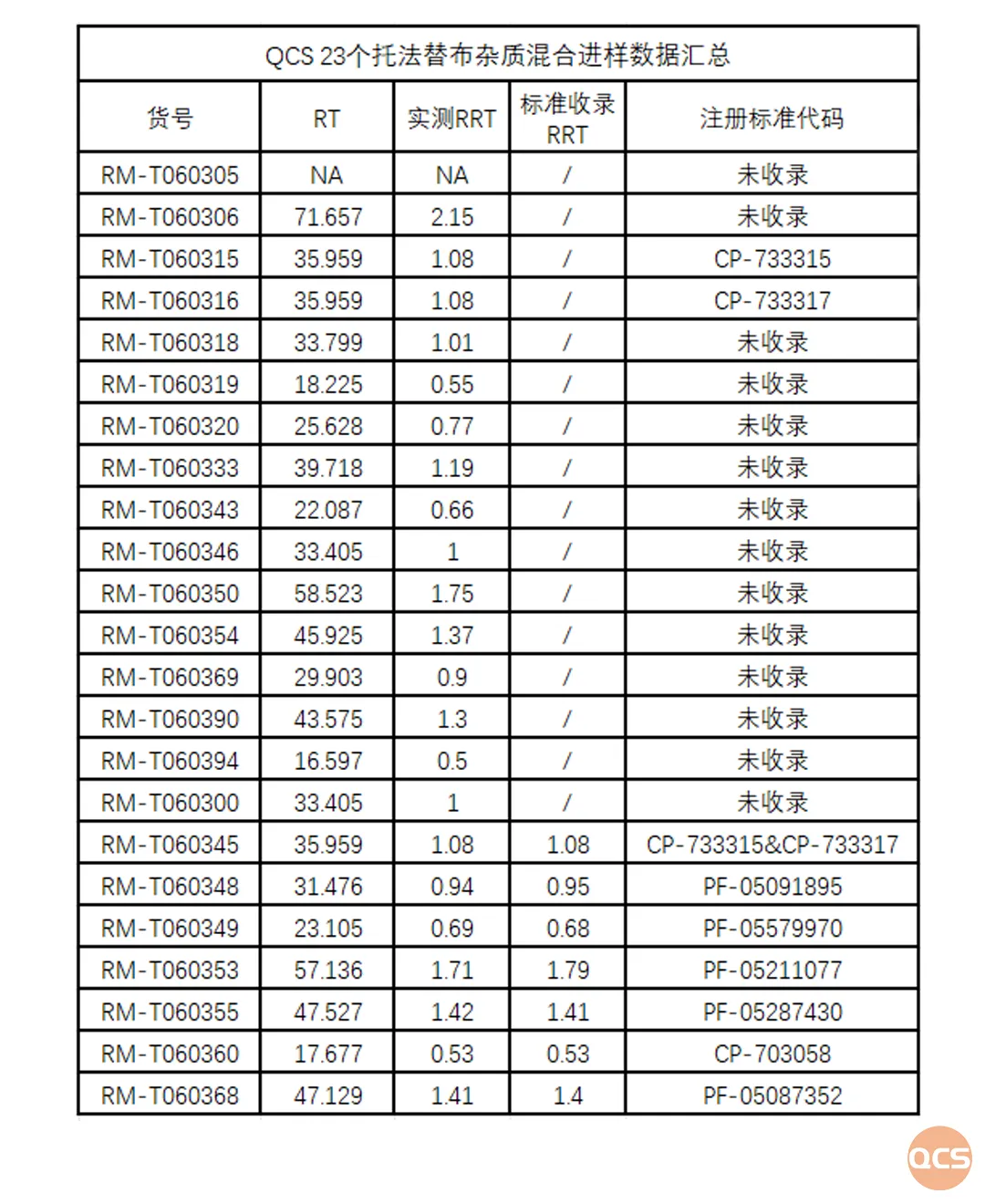
Figure 5: A comprehensive Chromatogram of 33 impurity profiles and data aggregation.
The purpose of this study was to conduct a comparative study of 23 products developed by the QCS Standard Materials Research Center under uniform chromatographic conditions. As shown in Figure 2, simultaneous injection of 23 impurities under the same chromatographic conditions would cause some impurities to co-elute and not be effectively separated, such as RM-T0603(15&16&45) and RM-T0603(00&46), which could not be effectively separated.
Because product RM-T060315 (CP-733315, tofacitinib RS isomer) and RM-T060316 (CP-733317, tofacitinib SR isomer) are a pair of enantiomers, and RM-T060345 (CP-733315 & CP-733317) is a mixture of them and is a non-enantiomeric form of tofacitinib, so our R&D center developed a method to separate them specifically. The separation chromatogram is shown in Figure 6, and the data summary is shown in Figure 7.
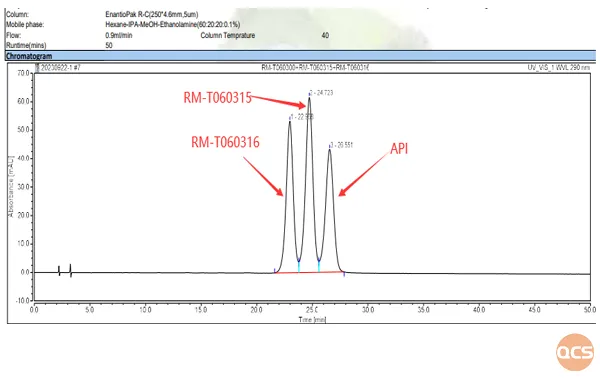
Figure 6: Chromatogram for the separation of chiral impurities in a mixture
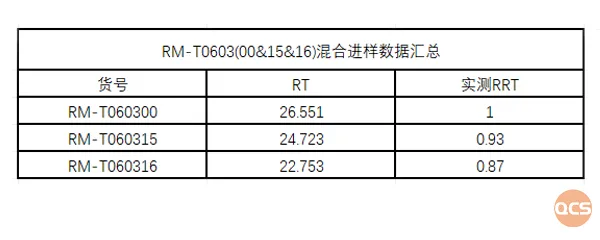
Figure 7: Summary of chiral separation data for RM-T060315 and RM-T060316
From the above data, it can be seen that multi-sample injection may cause certain interference on the chromatographic results. In order to provide better chromatographic reference information, the QCS Standard Materials R&D Center conducted additional separate injection tests for the 23 impurity products selected for this study. By combining the results of the separate sample tests and the mixture sample tests, we hope to provide more comprehensive reference information for the study of tofacitinib impurities. If needed, please contact our marketing team or the corresponding sales representative to obtain the separate chromatographic results for the impurity product. See Figure 8.
Figure 8: Summary of individual injection data for 23 impurities of tofacitinib
If there is a product of interest to you that is not covered in this study, please feel free to discuss it with us.
Long press the QR code to view a complete list of impurities!


Introduction: Today, we will discuss the impurities study of tofacitinib citrate tablets, a popular drug for rheumatoid arthritis treatment produced by Pfizer. Currently, there are 34 registration approvals for tofacitinib products and raw materials in China, including 4 approvals for finished products and 30 approvals for raw materials. Chinese companies such as ZHEJIANG HISUN PHARMACEUTICAL Co., LTD. have developed generic versions of the drug and have passed the consistency evaluation.
Tofacitinib citrate is the first JAK pathway inhibitor with a novel oral protein tyrosine kinase inhibitor mechanism, which can regulate the specific signal transduction pathway of JAK and prevent the phosphorylation and activation of signal transduction factors and transcription activators (STAT).
The official QCS website currently lists over 99 impurities of tofacitinib (Scan the QR code at the end of this article for the complete list of impurities). Our center has conducted relevant research on the primary impurities of tofacitinib, aligning with the import registration standard for Citric Acid Tofacitinib Tablets (Standard No. JX20130251). The structural information for both raw materials and specific customer-focused impurities is detailed in Figure 1.

Figure 1: List of Key Impurities for Tofacitinib Customers
Based on the substance method outlined in the import registration standard for Tofacatib Citrate Tablets (Standard No. JX20130251), our center primarily conducted qualitative localization research on 22 specific impurities, with detailed chromatographic data presented in FIG. 2 and FIG. 3.

Figure 2: Location chromatogram and data summary of 22 impurities in tofacitinib

Figure 3: Location chromatogram and data summary of 8 specified impurities (excluding impurities with code PF-05198213) in the Tofacatib API and import registration standard.
In this investigation, QCS R&D Center meticulously selected 23 impurity products from its library for comprehensive analysis and determined their respective relative retention times (refer to Figure 4). Despite variations between our chromatographic column's stationary phase and that of established standards, we utilized seven impurities coded under PF-05198213 from Tofacitile citrate tablet's import registration standard as reference points. The measured relative retention times closely aligned with those specified in these standards, indicating a high degree of consistency. Detailed data is available in Figure 4.

Figure 4: Code impurity-related standards and verification data in import registration standards

Figure 5: A comprehensive Chromatogram of 33 impurity profiles and data aggregation.
The purpose of this study was to conduct a comparative study of 23 products developed by the QCS Standard Materials Research Center under uniform chromatographic conditions. As shown in Figure 2, simultaneous injection of 23 impurities under the same chromatographic conditions would cause some impurities to co-elute and not be effectively separated, such as RM-T0603(15&16&45) and RM-T0603(00&46), which could not be effectively separated.
Because product RM-T060315 (CP-733315, tofacitinib RS isomer) and RM-T060316 (CP-733317, tofacitinib SR isomer) are a pair of enantiomers, and RM-T060345 (CP-733315 & CP-733317) is a mixture of them and is a non-enantiomeric form of tofacitinib, so our R&D center developed a method to separate them specifically. The separation chromatogram is shown in Figure 6, and the data summary is shown in Figure 7.

Figure 6: Chromatogram for the separation of chiral impurities in a mixture

Figure 7: Summary of chiral separation data for RM-T060315 and RM-T060316
From the above data, it can be seen that multi-sample injection may cause certain interference on the chromatographic results. In order to provide better chromatographic reference information, the QCS Standard Materials R&D Center conducted additional separate injection tests for the 23 impurity products selected for this study. By combining the results of the separate sample tests and the mixture sample tests, we hope to provide more comprehensive reference information for the study of tofacitinib impurities. If needed, please contact our marketing team or the corresponding sales representative to obtain the separate chromatographic results for the impurity product. See Figure 8.

Figure 8: Summary of individual injection data for 23 impurities of tofacitinib
If there is a product of interest to you that is not covered in this study, please feel free to discuss it with us.
Long press the QR code to view a complete list of impurities!


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号