Time:2022-12-01
Introduction: This issue will take the impurity ER-001132089-000 (nitrogen oxide) of Avatrombopag as an example to analyze the ten original code impurities in the original research standard.
Avatrombopag Maleate Tablets (trade name: Doptelet) received marketing approval from the US Food and Drug Administration (FDA) in 2018 for adult patients with CLD associated thrombocytopenia. Avatrombopag is the world's first FDA approved oral thrombopoietin receptor agonist (TPO-RA) for CLD associated thrombocytopenia, and also the first small molecule innovative drug introduced by Fosun Pharma. As the first domestic drug for the treatment of chronic liver disease (CLD) - related thrombocytopenia, the launch of Avatrombopag in China fills the medication gap in this field and introduces a globally leading "strong, long-lasting, safe, and convenient" new diagnosis and treatment plan for Chinese patients with CLD - related thrombocytopenia.
According to the data obtained by the QCS Standard Reference Material Research and Development Center, there are ten code impurities in the raw material of Avatrombopag, and the specific impurity code names are shown in Figure 1.
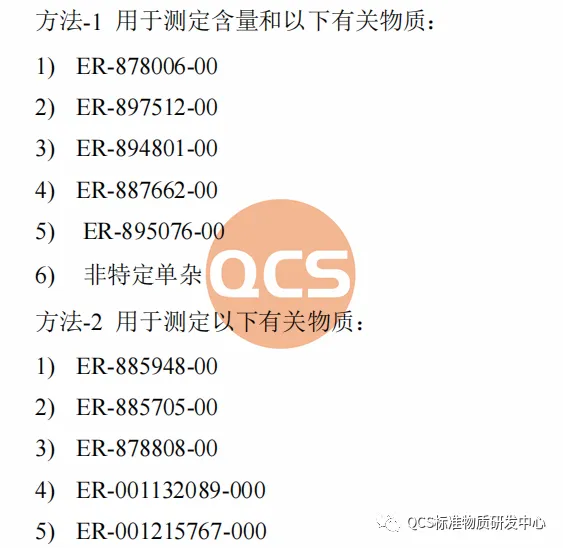
Figure 1: Impurity information in original research code
From Figure 1, it can be seen that the entire impurity study used two sets of liquid chromatography methods to identify different impurities. The specific methods, identification of impurities, and relative retention time (RRT) are shown in Figures 2 and 3:
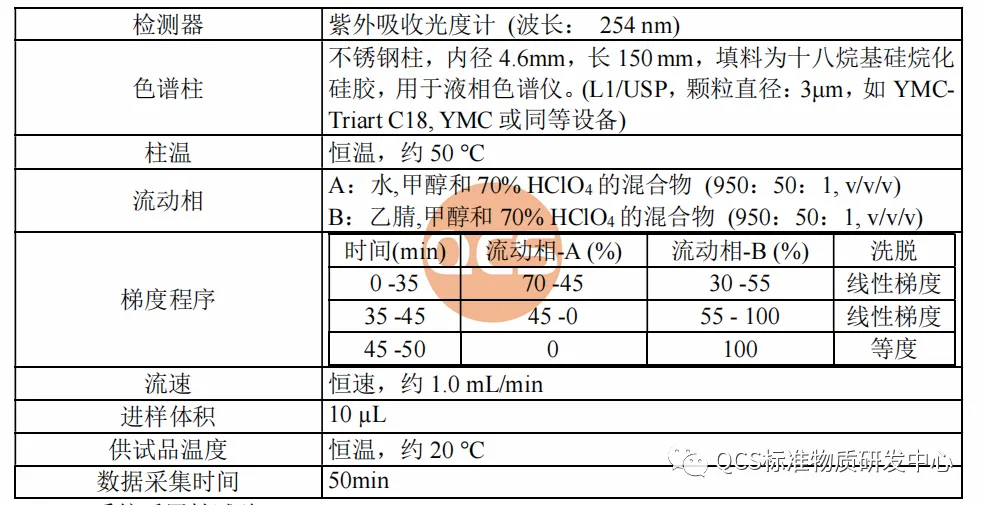
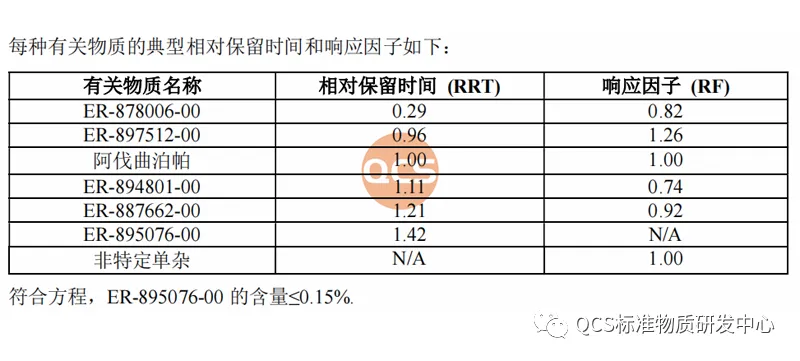
Figure 2: Code Impurities and Relative Retention Time under Method 1
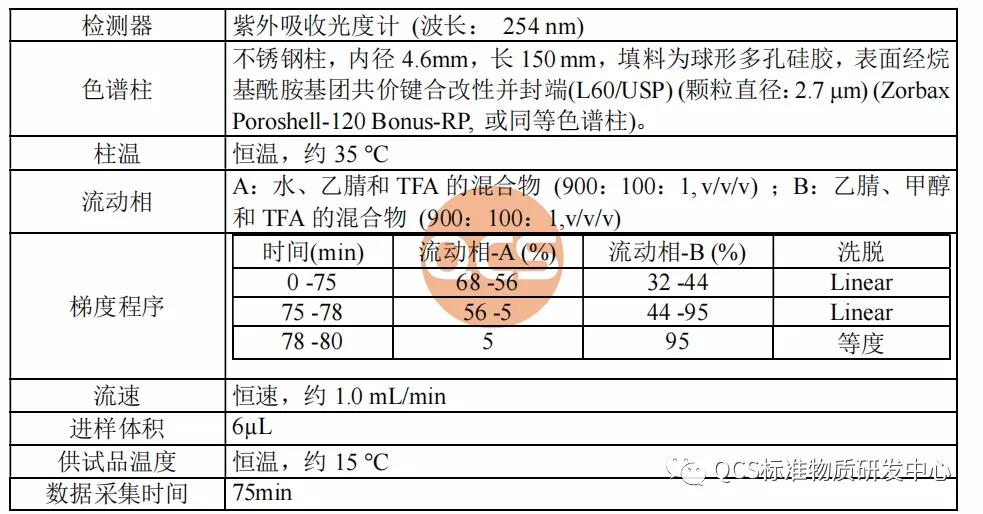
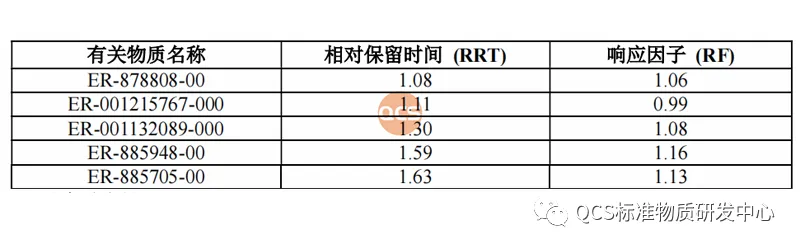
Figure 3: Impurity codes and relative retention time under Method 2
The code impurity ER-001132089-000 we are going to share today is actually the nitrogen oxide impurity of Avatrombopag (QCS item number: RM-A220103), corresponding to impurity III in the import standard of Avatrombopag maleate tablets, with a relative retention time (RRT) of 1.30. Impurity I in the import standard of the formulation corresponds to the original research code ER-887662-00 (QCS item number: RM-A220101, RRT of 1.21), and impurity II in the import standard of the formulation corresponds to the original research code ER-878808-00 (QCS item number: RM-A220102, RRT of 1.08). The structural formulas of the above three impurities are detailed in the National Medical Products Administration Import Drug Registration Standard (Standard Number: JX20190262), Customers in need can contact us through the backend.
Our center has followed method 2 to locate impurities, as shown in Figure 4 for specific information
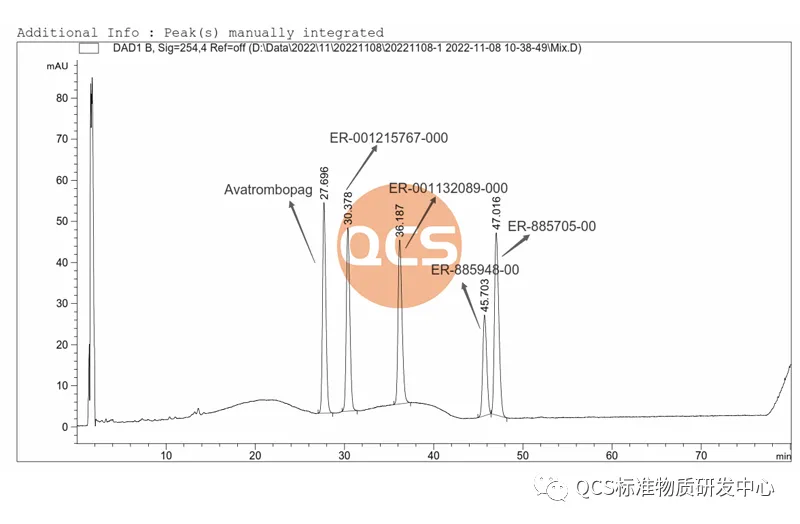
Figure 4: Chromatogram of code impurity localization under Method 2
Except for ER-001132089-000 (QCS Goods No.: RM-A220103), There are also three code impurities: ER-885948-00, ER-885705-00 and ER-001215767-000, among them, the two impurities ER-885948-00 and ER-885705-00 are relatively easy to infer, the corresponding relationship with QCS products is ER-885948-00 (corresponding QCS item number: RM-A220129), ER-885705-00 (corresponding QCS item number: RM-A220134), The impurity structure formulas of these two codes are detailed in Figure 5. Firstly, the unique numbering of this impurity makes it easy to be classified incorrectly; Secondly, the source of impurities is special, and it is necessary to have a deep understanding of the connection between CMA and CQA of the product. If customer has encountered the same problem as us, weclome to contact the editor to request a sample for verification.
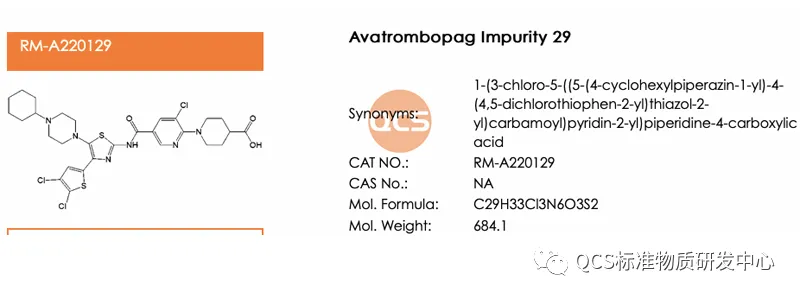
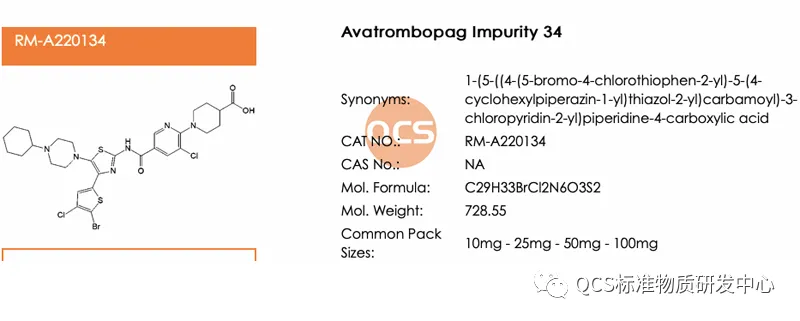
Figure 5: Structural information of ER-885948-00 (item number: RM-A220129) and ER-885705-00 (item number: RM-A220134)
Customer interaction: Recently, we received feedback from a customer that they want to study impurities on the pyridine ring where N is oxidized but nitrogen on the pyridine ring is not oxidized (as shown in Figure 6). From the logic of synthesis, if the impurity is generated from long-term stability experiments, it indicates that it is a real oxidized impurity. Therefore, only the most easily oxidized site can produce oxide (ER-001132089-000) instead of Figure 6. The reason is that it is close to the benzene ring, with low electron cloud density and cannot be oxidized. There are no lone pair electrons, forming a π bond, which is theoretically impossible to produce. If it is artificially synthesized impurities, including nitrogen on pyridine, it will also be oxidized, so the research significance is not significant!
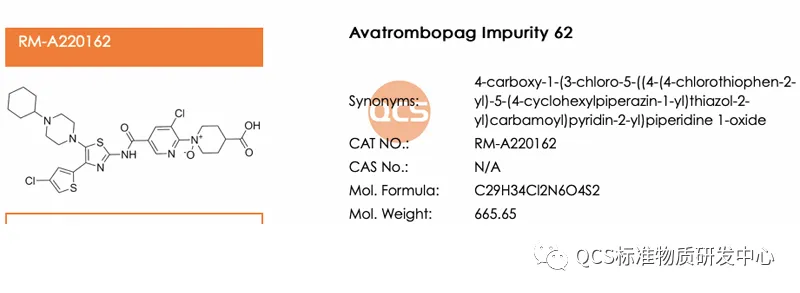
Figure 6: The structural formula of the product formed by the oxidation of nitrogen on the pyridine ring
Figure 7 shows two simple code impurity localization maps made according to the chromatographic conditions in Method 1, ER-878006-00 (QCS item number: RM-A220147, relative retention time 0.29) and ER-887662-00 (QCS item number: RM-A220101, relative retention time 1.21). The feedback from the figures shows that the relative retention time is consistent with the original research standard.
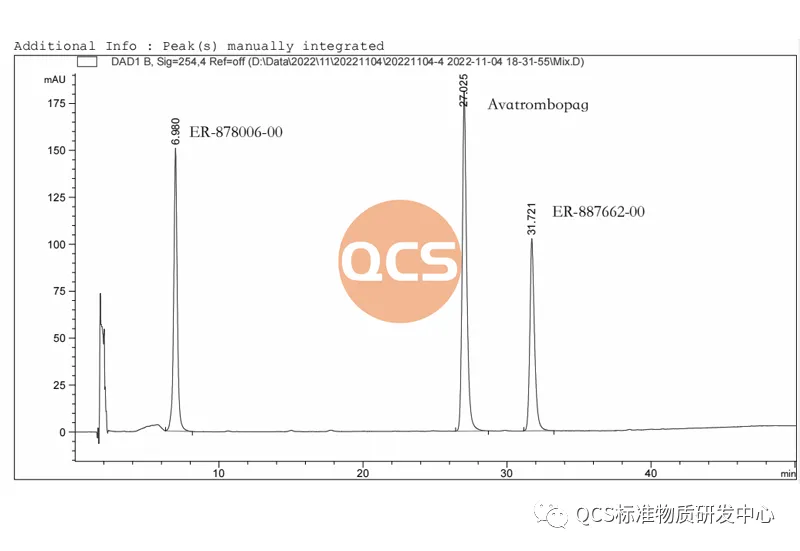
Figure 7: Two code impurity localization maps under Method 1
Finally, there is the trimer in the original research, corresponding to impurity code ER-895076-00 (QCS item number: RM-A220149), relative retention time (RRT 1.42), and structural formula shown in Figure 8. The trimer should be easy to infer, but synthesis is particularly difficult with poor solubility. Our center is making every effort to combat trimer, and we welcome everyone to request trimer samples from our center when the time comes.

Figure 8: Trimer impurity ER-895076-00 structural formula (QCS item number: RM-A220149)
This article has basically explained all ten code impurities. If any customers have doubts about code impurities, they can directly contact our backend to obtain the structural information of all 10 code impurities. Finally, thank you for your continuous support.

Introduction: This issue will take the impurity ER-001132089-000 (nitrogen oxide) of Avatrombopag as an example to analyze the ten original code impurities in the original research standard.
Avatrombopag Maleate Tablets (trade name: Doptelet) received marketing approval from the US Food and Drug Administration (FDA) in 2018 for adult patients with CLD associated thrombocytopenia. Avatrombopag is the world's first FDA approved oral thrombopoietin receptor agonist (TPO-RA) for CLD associated thrombocytopenia, and also the first small molecule innovative drug introduced by Fosun Pharma. As the first domestic drug for the treatment of chronic liver disease (CLD) - related thrombocytopenia, the launch of Avatrombopag in China fills the medication gap in this field and introduces a globally leading "strong, long-lasting, safe, and convenient" new diagnosis and treatment plan for Chinese patients with CLD - related thrombocytopenia.
According to the data obtained by the QCS Standard Reference Material Research and Development Center, there are ten code impurities in the raw material of Avatrombopag, and the specific impurity code names are shown in Figure 1.

Figure 1: Impurity information in original research code
From Figure 1, it can be seen that the entire impurity study used two sets of liquid chromatography methods to identify different impurities. The specific methods, identification of impurities, and relative retention time (RRT) are shown in Figures 2 and 3:


Figure 2: Code Impurities and Relative Retention Time under Method 1


Figure 3: Impurity codes and relative retention time under Method 2
The code impurity ER-001132089-000 we are going to share today is actually the nitrogen oxide impurity of Avatrombopag (QCS item number: RM-A220103), corresponding to impurity III in the import standard of Avatrombopag maleate tablets, with a relative retention time (RRT) of 1.30. Impurity I in the import standard of the formulation corresponds to the original research code ER-887662-00 (QCS item number: RM-A220101, RRT of 1.21), and impurity II in the import standard of the formulation corresponds to the original research code ER-878808-00 (QCS item number: RM-A220102, RRT of 1.08). The structural formulas of the above three impurities are detailed in the National Medical Products Administration Import Drug Registration Standard (Standard Number: JX20190262), Customers in need can contact us through the backend.
Our center has followed method 2 to locate impurities, as shown in Figure 4 for specific information

Figure 4: Chromatogram of code impurity localization under Method 2
Except for ER-001132089-000 (QCS Goods No.: RM-A220103), There are also three code impurities: ER-885948-00, ER-885705-00 and ER-001215767-000, among them, the two impurities ER-885948-00 and ER-885705-00 are relatively easy to infer, the corresponding relationship with QCS products is ER-885948-00 (corresponding QCS item number: RM-A220129), ER-885705-00 (corresponding QCS item number: RM-A220134), The impurity structure formulas of these two codes are detailed in Figure 5. Firstly, the unique numbering of this impurity makes it easy to be classified incorrectly; Secondly, the source of impurities is special, and it is necessary to have a deep understanding of the connection between CMA and CQA of the product. If customer has encountered the same problem as us, weclome to contact the editor to request a sample for verification.


Figure 5: Structural information of ER-885948-00 (item number: RM-A220129) and ER-885705-00 (item number: RM-A220134)
Customer interaction: Recently, we received feedback from a customer that they want to study impurities on the pyridine ring where N is oxidized but nitrogen on the pyridine ring is not oxidized (as shown in Figure 6). From the logic of synthesis, if the impurity is generated from long-term stability experiments, it indicates that it is a real oxidized impurity. Therefore, only the most easily oxidized site can produce oxide (ER-001132089-000) instead of Figure 6. The reason is that it is close to the benzene ring, with low electron cloud density and cannot be oxidized. There are no lone pair electrons, forming a π bond, which is theoretically impossible to produce. If it is artificially synthesized impurities, including nitrogen on pyridine, it will also be oxidized, so the research significance is not significant!

Figure 6: The structural formula of the product formed by the oxidation of nitrogen on the pyridine ring
Figure 7 shows two simple code impurity localization maps made according to the chromatographic conditions in Method 1, ER-878006-00 (QCS item number: RM-A220147, relative retention time 0.29) and ER-887662-00 (QCS item number: RM-A220101, relative retention time 1.21). The feedback from the figures shows that the relative retention time is consistent with the original research standard.

Figure 7: Two code impurity localization maps under Method 1
Finally, there is the trimer in the original research, corresponding to impurity code ER-895076-00 (QCS item number: RM-A220149), relative retention time (RRT 1.42), and structural formula shown in Figure 8. The trimer should be easy to infer, but synthesis is particularly difficult with poor solubility. Our center is making every effort to combat trimer, and we welcome everyone to request trimer samples from our center when the time comes.

Figure 8: Trimer impurity ER-895076-00 structural formula (QCS item number: RM-A220149)
This article has basically explained all ten code impurities. If any customers have doubts about code impurities, they can directly contact our backend to obtain the structural information of all 10 code impurities. Finally, thank you for your continuous support.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号