Time:2023-02-10
Introduction: Prior to this year's Spring Festival, domestic epidemic prevention measures were fully relaxed, leading to a surge in global interest for ibuprofen. This drug, characterized by its timely relevance, quickly gained widespread attention among the population—an outcome that was somewhat unexpected. Reflecting on my graduation internship in 2009 at a pharmaceutical research and development company, I recall that they were already engaged in the formulation of orange suspension at that time. In fact, even before the COVID-19 pandemic heightened its visibility, ibuprofen had long been established as an essential antipyretic medication for households, particularly those with children. Therefore, today I would like to present the impurities associated with ibuprofen developed by our company.
Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) that serves as an antipyretic and analgesic. By inhibiting cyclooxygenase, it effectively reduces the synthesis of prostaglandins, thereby producing both analgesic and anti-inflammatory effects. Its antipyretic action is mediated through the hypothalamic thermoregulatory center. The sustained interest from generic drug companies in developing various formulations of ibuprofen can be attributed to its proven efficacy and the availability of multiple dosage forms, including ibuprofen tablets, suspensions, arginine tablets, granules, among others currently on the market.
In alignment with relevant substance specifications outlined in the European Pharmacopoeia (EP), our center has conducted localization verification for several specific impurities referenced in these standards. The EP standards pertaining to substance testing items are illustrated in Figure 1.
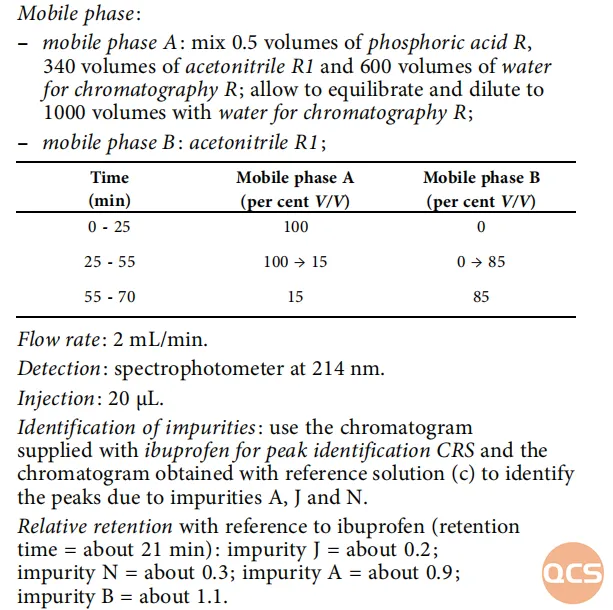
Figure 1: Ibuprofen (EP) related substance test items (Source: EP 10.0)
Given the straightforward structure of ibuprofen products, the process of structural verification is relatively uncomplicated. To enhance customer recognition, our center has conducted both separate and mixed injections in accordance with the relevant substance methods outlined in the European Pharmacopoeia. We are now pleased to share the diagram for the mixed injection (Figure 2) with you.
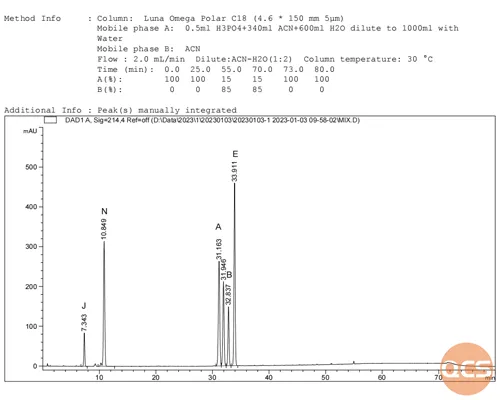
Figure 2: Chromatogram of mixed injection for ibuprofen EP impurity ABEJN
Data source: QCS Standard Materials Research and Development Center
As illustrated in the diagram above, while the primary peak retention time (RT) is postponed due to variations in column types, the overall relative retention time of impurities closely aligns with the relative retention time (RRT) of impurities specified in the EP standard. Refer to specific analytical data presented in Figure 3.
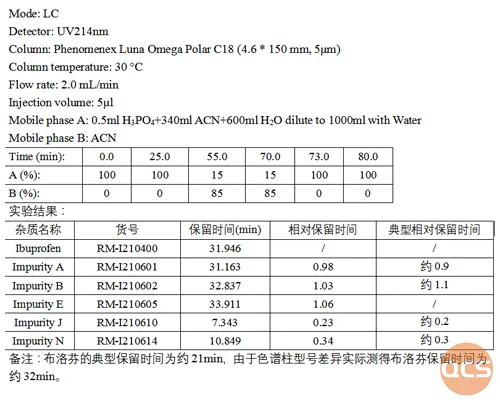
Figure 3: Chromatographic data analysis of the mixed injection of ibuprofen EP impurity ABEJN.
Our center has successfully developed a comprehensive set of impurities for ibuprofen EP, encompassing impurity A/B/C/D/E/F/G/H/I/J/K/L/M/N/O/P/Q/R/S. We welcome inquiries! Given the unique characteristics of ibuprofen as a product, it is important to note that most impurities are oil-based; therefore, customers should exercise caution regarding usage specifications and precise weighing.

Introduction: Prior to this year's Spring Festival, domestic epidemic prevention measures were fully relaxed, leading to a surge in global interest for ibuprofen. This drug, characterized by its timely relevance, quickly gained widespread attention among the population—an outcome that was somewhat unexpected. Reflecting on my graduation internship in 2009 at a pharmaceutical research and development company, I recall that they were already engaged in the formulation of orange suspension at that time. In fact, even before the COVID-19 pandemic heightened its visibility, ibuprofen had long been established as an essential antipyretic medication for households, particularly those with children. Therefore, today I would like to present the impurities associated with ibuprofen developed by our company.

Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) that serves as an antipyretic and analgesic. By inhibiting cyclooxygenase, it effectively reduces the synthesis of prostaglandins, thereby producing both analgesic and anti-inflammatory effects. Its antipyretic action is mediated through the hypothalamic thermoregulatory center. The sustained interest from generic drug companies in developing various formulations of ibuprofen can be attributed to its proven efficacy and the availability of multiple dosage forms, including ibuprofen tablets, suspensions, arginine tablets, granules, among others currently on the market.
In alignment with relevant substance specifications outlined in the European Pharmacopoeia (EP), our center has conducted localization verification for several specific impurities referenced in these standards. The EP standards pertaining to substance testing items are illustrated in Figure 1.

Figure 1: Ibuprofen (EP) related substance test items (Source: EP 10.0)
Given the straightforward structure of ibuprofen products, the process of structural verification is relatively uncomplicated. To enhance customer recognition, our center has conducted both separate and mixed injections in accordance with the relevant substance methods outlined in the European Pharmacopoeia. We are now pleased to share the diagram for the mixed injection (Figure 2) with you.

Figure 2: Chromatogram of mixed injection for ibuprofen EP impurity ABEJN
Data source: QCS Standard Materials Research and Development Center
As illustrated in the diagram above, while the primary peak retention time (RT) is postponed due to variations in column types, the overall relative retention time of impurities closely aligns with the relative retention time (RRT) of impurities specified in the EP standard. Refer to specific analytical data presented in Figure 3.

Figure 3: Chromatographic data analysis of the mixed injection of ibuprofen EP impurity ABEJN.
Our center has successfully developed a comprehensive set of impurities for ibuprofen EP, encompassing impurity A/B/C/D/E/F/G/H/I/J/K/L/M/N/O/P/Q/R/S. We welcome inquiries! Given the unique characteristics of ibuprofen as a product, it is important to note that most impurities are oil-based; therefore, customers should exercise caution regarding usage specifications and precise weighing.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号