Time:2023-10-30
Introduction: Today, I will share a study on impurities in the drug Febuxostat that I am very familiar with. Febuxostat tablets can be said to have become an essential medicine for home travel.

Figure 1: Just identify the ones with a checkmark (√) when buying medicine
Febuxostat is a novel xanthine oxidase inhibitor that lowers blood uric acid levels by reducing uric acid production. In February 2009, Febuxostat was approved by the US FDA for the treatment of adult gout under the brand name Ulolic. In 2013, Febuxostat was launched in China.
At present, the QCS official website has included over 110 impurities of Febuxostat (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the Febuxostat content determination method in the literature and conducted relevant research on several specific impurities of Febuxostat. The specific impurity structure information is shown in Figure 2:
:
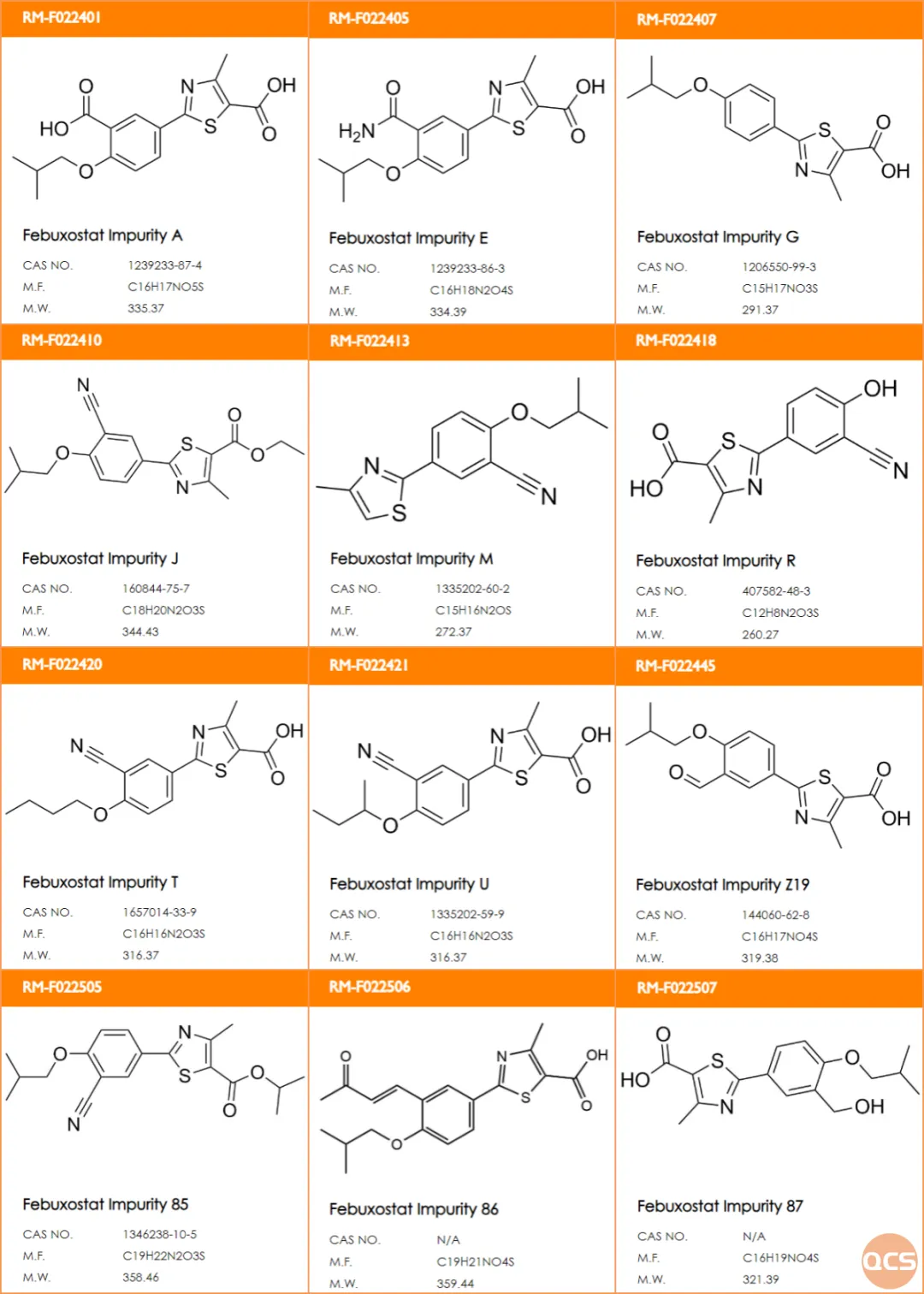
Figure 2: List of key research impurities in Febuxostat
Data source: QCS Standard Material Research and Development Center
Febuxostat, the current available reference materials include information related to import registration standards and information related to the Japanese Medical Device Evaluation & Approval Agency (PMDA). The import registration standard includes two impurities: RM-F022465 and RM-F022413. The IF document of Donghe Jingpin Co., Ltd. published by PMDA includes impurities RM-F022405 and RM-F022413.
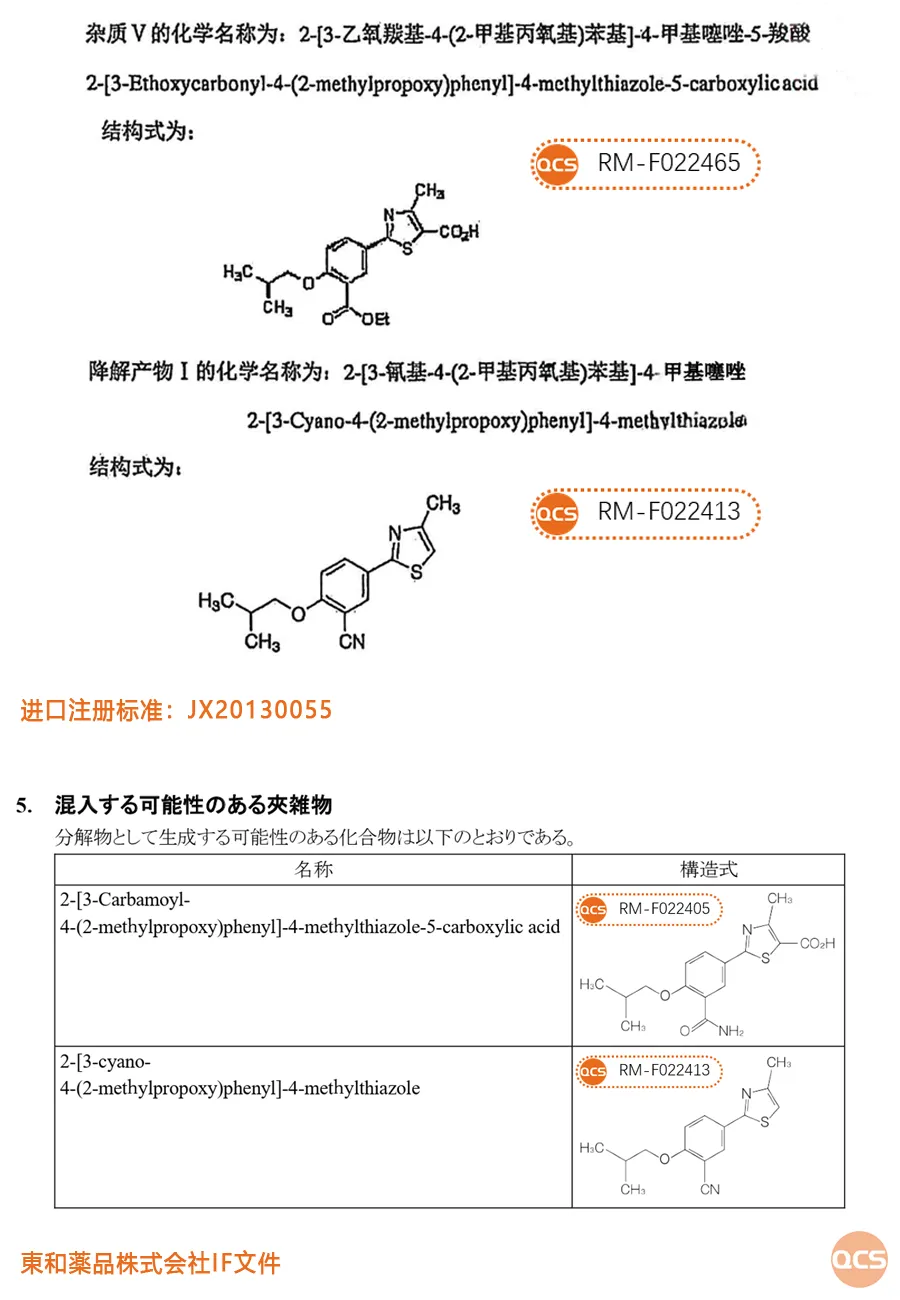
Figure 3: Evaluation materials related to Febuxostat and their inclusion impurity list
Compared to the above import registration standards and PMDA related document disclosures, domestic pharmaceutical companies have conducted relatively sufficient research on impurities in this product, studying dozens of impurities. This article combines the internal control standards of domestic Febuxostat related raw material enterprises, and selects 12 common main impurities for sharing, of which 5 impurities have been included in the quality standards of domestic enterprises. The QCS Standard Reference Material Research and Development Center has conducted research on the impurity samples and API standards mentioned above. Based on relevant literature, liquid chromatography was used to locate them. Today, we will mainly share several impurity localization data that have been included in the standards for your reference, as shown in Figure 4:
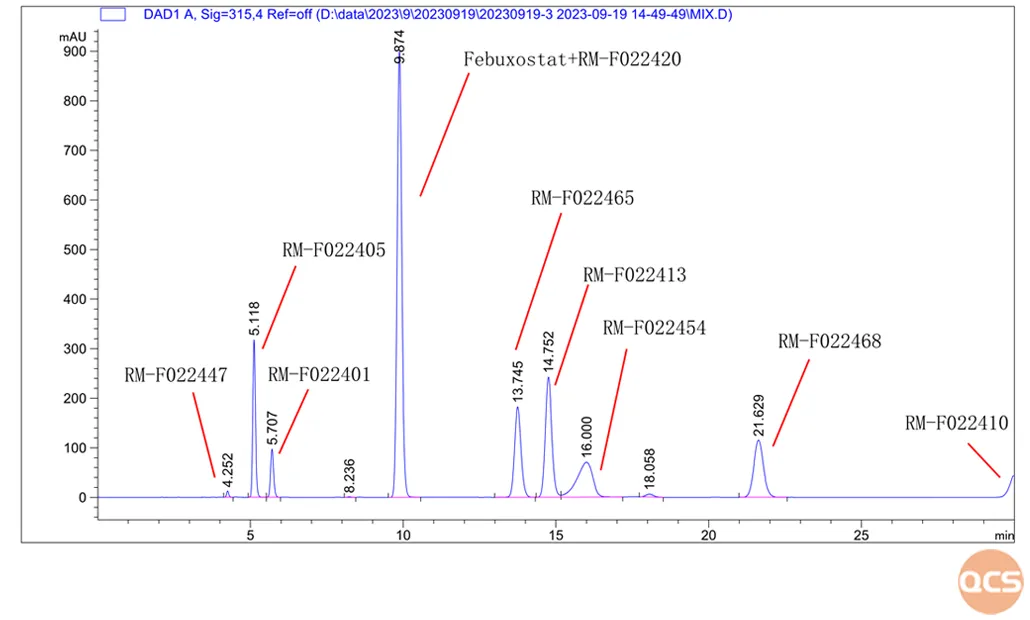
Figure 4: Impurity localization data
Data source: QCS Standard Material Research and Development Center
From Figure 4, it can be seen that the effective separation of Febuxostat and impurity RM-F022420 cannot be achieved, and they basically overlap completely. It is speculated that the reason is that the two products are isomers of each other, and the difference is very small, which is the difference between isobutyl and n-butyl on the side chain. The individual injection data and product structure formula are shown in Figure 5:
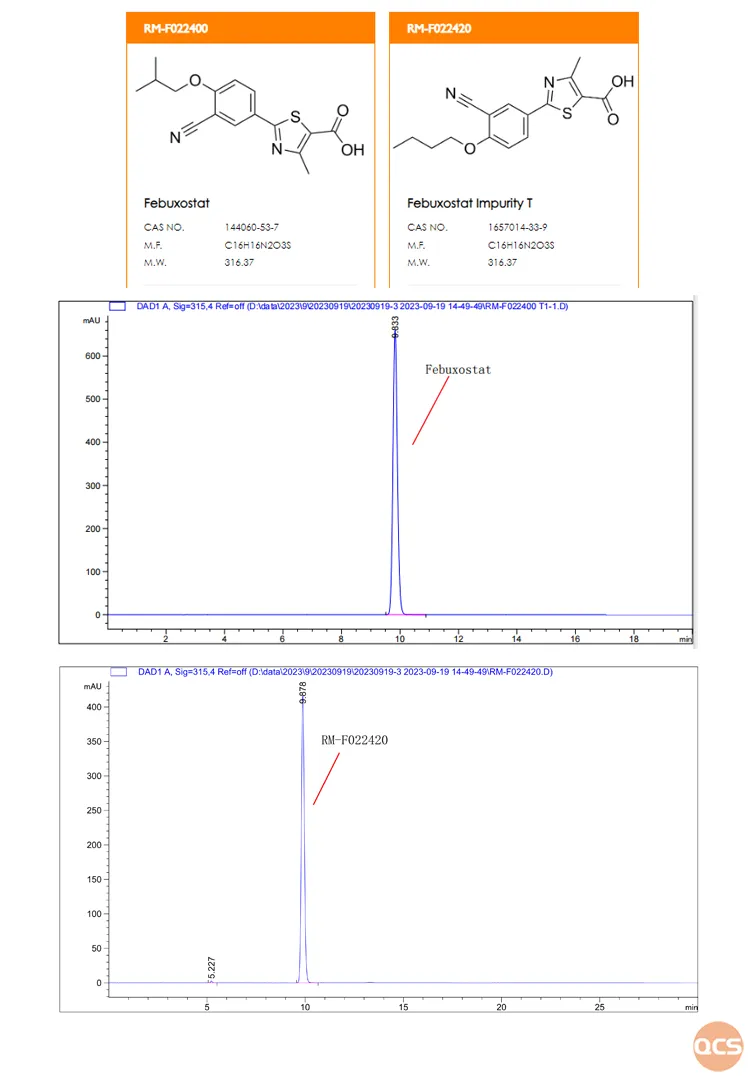
Figure 5: Structure and individual injection data of Febuxostat and RM-F022420
Data source: QCS Standard Material Research and Development Center
Febuxostat has not yet been included in the official pharmacopoeia, and there are currently no legal analysis and testing conditions. The QCS Standard Reference Material Research and Development Center has developed a liquid chromatography analysis method based on multiple literature sources. From the data, it can be seen that it is difficult to effectively separate API and n-butyl impurities (RM-F022420, Febuxostat Impurity T) under this chromatographic condition. The content determination method used for related substance detection has defects and needs to be optimized in order to establish a more reasonable method for related substance determination (customers are welcome to discuss further method optimization with our center).
Please note that the literature on Febuxostat is as follows. If you need the original text, please contact the editor in the background ~

Long press to recognize the QR code and view the list of all impurities!


Introduction: Today, I will share a study on impurities in the drug Febuxostat that I am very familiar with. Febuxostat tablets can be said to have become an essential medicine for home travel.

Figure 1: Just identify the ones with a checkmark (√) when buying medicine
Febuxostat is a novel xanthine oxidase inhibitor that lowers blood uric acid levels by reducing uric acid production. In February 2009, Febuxostat was approved by the US FDA for the treatment of adult gout under the brand name Ulolic. In 2013, Febuxostat was launched in China.
At present, the QCS official website has included over 110 impurities of Febuxostat (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the Febuxostat content determination method in the literature and conducted relevant research on several specific impurities of Febuxostat. The specific impurity structure information is shown in Figure 2:
:

Figure 2: List of key research impurities in Febuxostat
Data source: QCS Standard Material Research and Development Center
Febuxostat, the current available reference materials include information related to import registration standards and information related to the Japanese Medical Device Evaluation & Approval Agency (PMDA). The import registration standard includes two impurities: RM-F022465 and RM-F022413. The IF document of Donghe Jingpin Co., Ltd. published by PMDA includes impurities RM-F022405 and RM-F022413.

Figure 3: Evaluation materials related to Febuxostat and their inclusion impurity list
Compared to the above import registration standards and PMDA related document disclosures, domestic pharmaceutical companies have conducted relatively sufficient research on impurities in this product, studying dozens of impurities. This article combines the internal control standards of domestic Febuxostat related raw material enterprises, and selects 12 common main impurities for sharing, of which 5 impurities have been included in the quality standards of domestic enterprises. The QCS Standard Reference Material Research and Development Center has conducted research on the impurity samples and API standards mentioned above. Based on relevant literature, liquid chromatography was used to locate them. Today, we will mainly share several impurity localization data that have been included in the standards for your reference, as shown in Figure 4:

Figure 4: Impurity localization data
Data source: QCS Standard Material Research and Development Center
From Figure 4, it can be seen that the effective separation of Febuxostat and impurity RM-F022420 cannot be achieved, and they basically overlap completely. It is speculated that the reason is that the two products are isomers of each other, and the difference is very small, which is the difference between isobutyl and n-butyl on the side chain. The individual injection data and product structure formula are shown in Figure 5:

Figure 5: Structure and individual injection data of Febuxostat and RM-F022420
Data source: QCS Standard Material Research and Development Center
Febuxostat has not yet been included in the official pharmacopoeia, and there are currently no legal analysis and testing conditions. The QCS Standard Reference Material Research and Development Center has developed a liquid chromatography analysis method based on multiple literature sources. From the data, it can be seen that it is difficult to effectively separate API and n-butyl impurities (RM-F022420, Febuxostat Impurity T) under this chromatographic condition. The content determination method used for related substance detection has defects and needs to be optimized in order to establish a more reasonable method for related substance determination (customers are welcome to discuss further method optimization with our center).
Please note that the literature on Febuxostat is as follows. If you need the original text, please contact the editor in the background ~

Long press to recognize the QR code and view the list of all impurities!


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号