Time:2023-10-12
Introduction: At the end of September 2023, our company had the opportunity to participate International Symposium on Pharmaceutical Impurity Control hosted by USP. The main topic was the control of N-nitrosamine series impurities, a genotoxic impurity. Through the sharing of multiple domestic and foreign experts, I have benefited greatly. (Successfully concluded | International Symposium on Pharmaceutical Impurity Control 2023)

Figure 1: USP- Attendance certificate of International Symposium on Pharmaceutical Impurity Control 2023
Recently, we have received many inquiries from customers about N-nitrosamine impurities, whether it is approved varieties or varieties being replicated, whether it is intermediates or starting materials, there are various types. We would like to share with you the information on the recommended acceptable intake of nitrosamines impurities that has been publicly announced by the FDA. Some customers may not know where to search. The specific search website is as follows, long press to identify the QR code:

The specific query method is shown in Figure 2:
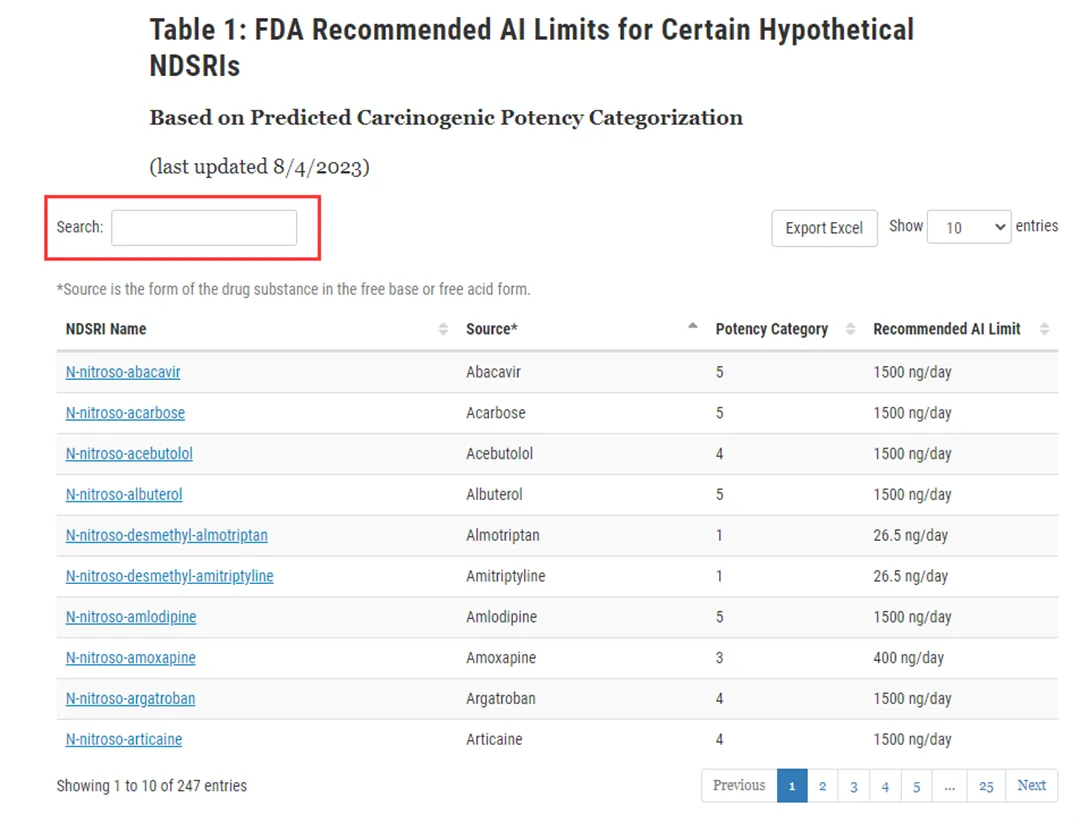
Figure 2: Specific query method and process
At present, the FDA has released a total of 247 nitrosamine genotoxic impurities. Based on the FDA's public information, our center has completed the synthesis of most genotoxic impurities and published them on the QCS official website( https://www.qcsrm.com/ )The specific query only requires entering the FDA genotoxic impurity name (in English) to complete the search, as shown in Figure 3:
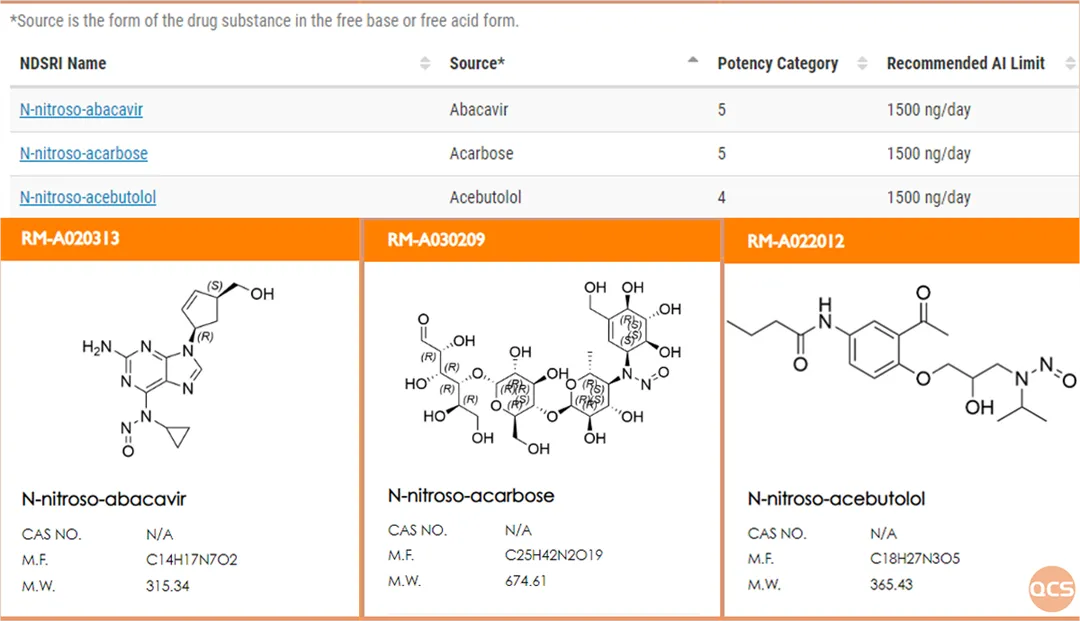
Figure 3: QCS collects information on N-nitrosamine impurities publicly disclosed by FDA
At the 2023 International Symposium on Pharmaceutical Impurity Control, the most frequently mentioned topic by experts is the establishment of scientific and reasonable analysis methods and monitoring tools to effectively control potential genotoxic impurities. But this job requires a lot of attention to detail. Director Qingsheng Zhang and his team at the China National Medical Products Administration are committed to establishing our own N-nitrosamine gene toxicity impurity library, similar to the work done by the FDA now, to provide basic data on nitrosamine related impurities for everyone. This is a project that benefits the people, and we hope that this system can be launched as soon as possible.
From the current client's consulting and procurement needs, there is a trend of everyone being picky about impurities at the beginning of the 16 year consistency evaluation. Customers often send over a dozen so-called genotoxic impurities for a single variety, from starting materials to intermediates, APIs, and formulation degradation. It feels like as long as there is - NH in the structure, there will definitely be N-NO production. Is there a possibility of over research on this? What is the mechanism of structure generation? Is there an objective condition for its occurrence? Is our method scientifically reasonable? This is all worth our careful consideration.

Introduction: At the end of September 2023, our company had the opportunity to participate International Symposium on Pharmaceutical Impurity Control hosted by USP. The main topic was the control of N-nitrosamine series impurities, a genotoxic impurity. Through the sharing of multiple domestic and foreign experts, I have benefited greatly. (Successfully concluded | International Symposium on Pharmaceutical Impurity Control 2023)

Figure 1: USP- Attendance certificate of International Symposium on Pharmaceutical Impurity Control 2023
Recently, we have received many inquiries from customers about N-nitrosamine impurities, whether it is approved varieties or varieties being replicated, whether it is intermediates or starting materials, there are various types. We would like to share with you the information on the recommended acceptable intake of nitrosamines impurities that has been publicly announced by the FDA. Some customers may not know where to search. The specific search website is as follows, long press to identify the QR code:

The specific query method is shown in Figure 2:

Figure 2: Specific query method and process
At present, the FDA has released a total of 247 nitrosamine genotoxic impurities. Based on the FDA's public information, our center has completed the synthesis of most genotoxic impurities and published them on the QCS official website( https://www.qcsrm.com/ )The specific query only requires entering the FDA genotoxic impurity name (in English) to complete the search, as shown in Figure 3:

Figure 3: QCS collects information on N-nitrosamine impurities publicly disclosed by FDA
At the 2023 International Symposium on Pharmaceutical Impurity Control, the most frequently mentioned topic by experts is the establishment of scientific and reasonable analysis methods and monitoring tools to effectively control potential genotoxic impurities. But this job requires a lot of attention to detail. Director Qingsheng Zhang and his team at the China National Medical Products Administration are committed to establishing our own N-nitrosamine gene toxicity impurity library, similar to the work done by the FDA now, to provide basic data on nitrosamine related impurities for everyone. This is a project that benefits the people, and we hope that this system can be launched as soon as possible.
From the current client's consulting and procurement needs, there is a trend of everyone being picky about impurities at the beginning of the 16 year consistency evaluation. Customers often send over a dozen so-called genotoxic impurities for a single variety, from starting materials to intermediates, APIs, and formulation degradation. It feels like as long as there is - NH in the structure, there will definitely be N-NO production. Is there a possibility of over research on this? What is the mechanism of structure generation? Is there an objective condition for its occurrence? Is our method scientifically reasonable? This is all worth our careful consideration.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号