Time:2023-08-03
Last week, in the article "Research Sharing on Impurities Related to Dysmenorrhea Medications - Elagolix", we mentioned that in the study of the raw materials and enantiomers of Elagolix, we found that there were double peaks in the liquid chromatography results, but the actual purity was actually very high. I think many friends have encountered this problem. Especially in the process of structural characterization, the colleagues of the Elagolix project must have been confused about why high-purity products always have numerous peaks when analyzed by nuclear magnetic resonance spectroscopy, and why the spectra of DMSO-d6, CD3OD, and D2O differ greatly, sometimes even showing two peaks on the high-performance liquid phase and sometimes showing one peak. Although for raw material pharmaceutical products, due to the abundance of standard information, all issues can be briefly addressed with the phrase 'consistent data standards', the research on related substances will bring great challenges
Due to the lack of comparable standard data, the structural characterization and qualitative and quantitative analysis of products require more self completion. For the molecules related to the Elagolix project, although multiple peaks and complex peaks appear in chromatography or nuclear magnetic resonance spectra, this does not mean that there is a problem with product quality. On the contrary, these results are determined by the chemical properties of the compound itself. For example, the special chromatographic results of Elagolix related substances are caused by the problem of hindered isomers. This article will take you to a detailed understanding of what hindered isomers are and what phenomena they may appear in chromatographic and other detection results.
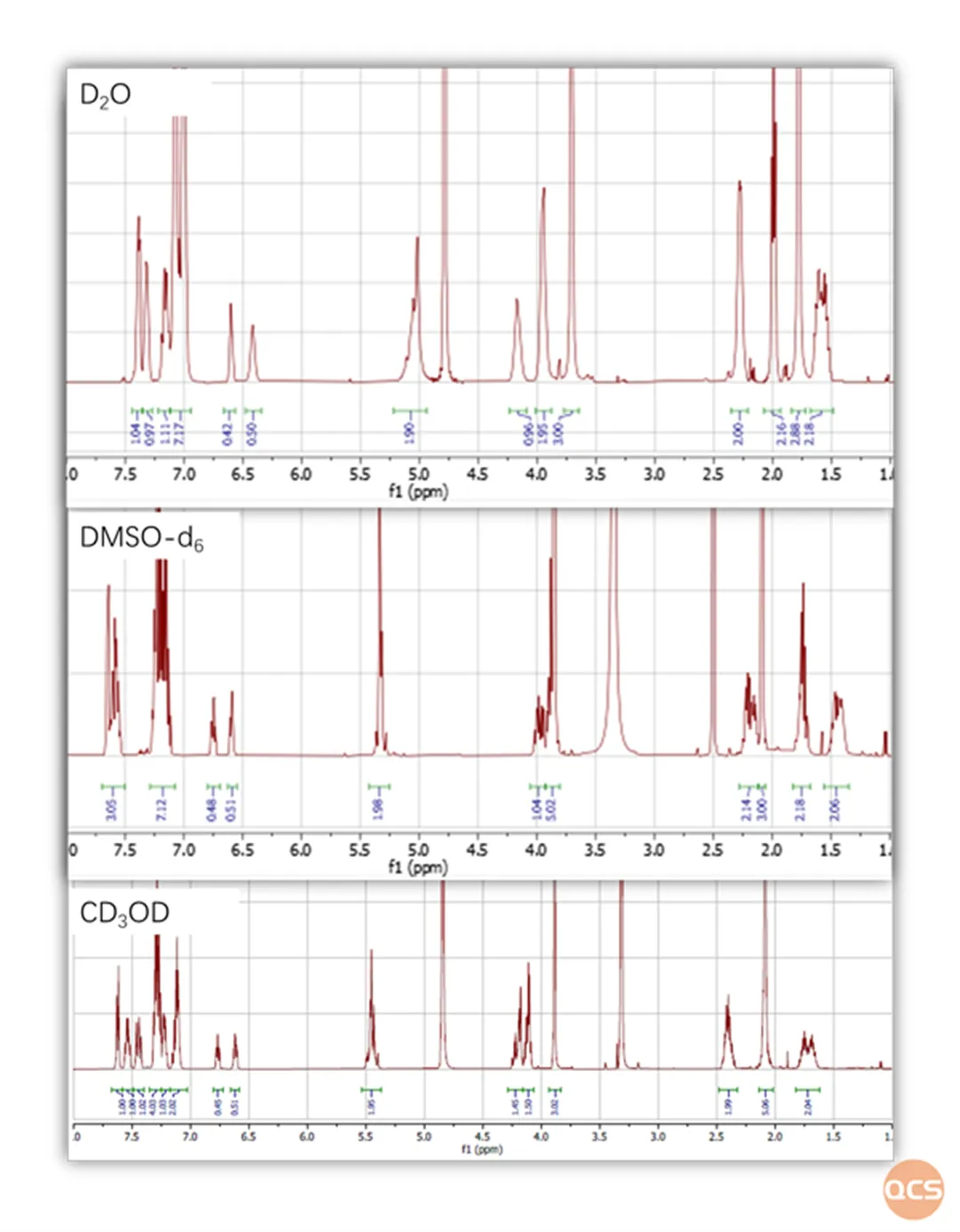
Figure 1: NMR spectra of Elagolix in different solvents under the influence of hindered isomers
Refractive isomers, also known as steric hindrance active isomers, are a class of optically active isomers containing chiral axes. Unlike most chiral compounds formed by asymmetric atoms, optically active isomers containing chiral axes do not necessarily require chemical conditions to convert to each other. Their molecules may form chemical equilibrium when heated to a certain energy. Compared to conformational isomers, hindered isomers must be relatively stable. According to Oki's definition, the half-life of the transformation between hindered isomers at a certain temperature should be greater than 1000 seconds (source: Oki, M; Topics in Stereochemistry 1983, 1)。
The hindered rotation isomer is different from classical new chemical entities, it is just two different conformations produced by the hindrance of chemical bond rotation in one molecule. Due to the fact that two isomers can be converted into each other but have a certain energy barrier, some hindered isomers can be separated by chromatography. Therefore, in chiral HPLC, there will be a double peak (as shown in Figure 2) in the hindered enantiomer of Elagolix.
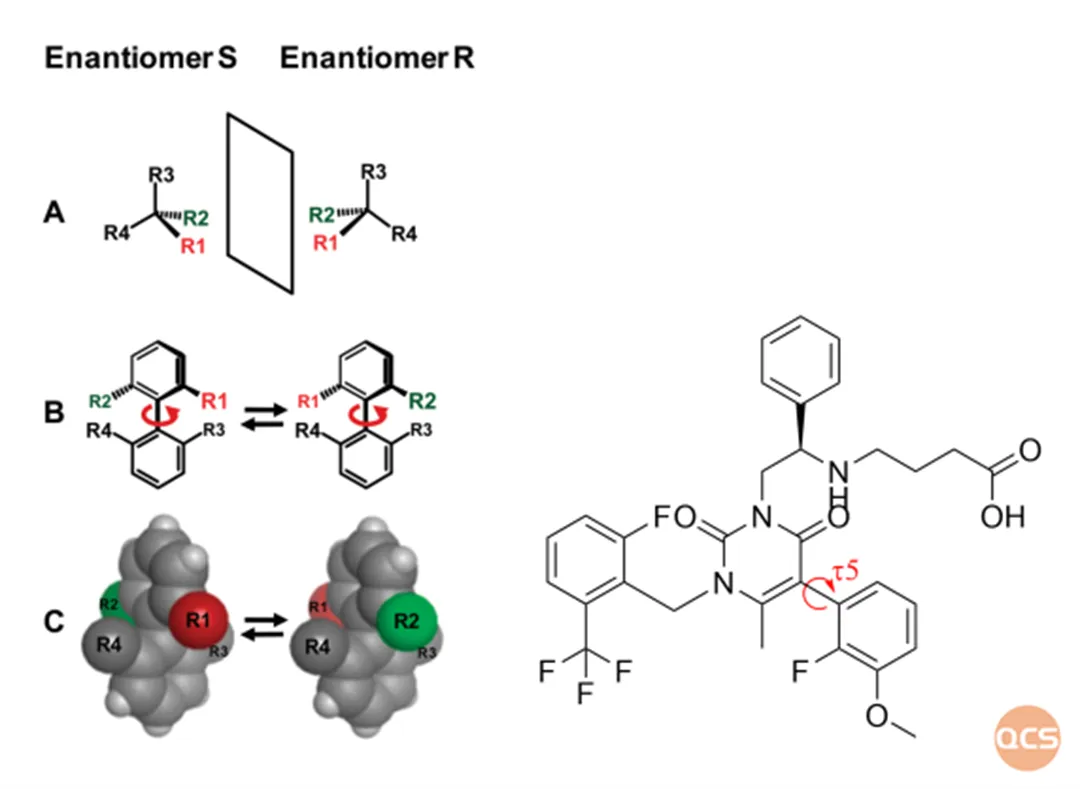
Figure 2: Schematic diagram of the structural formula and τ 5 chemical bond rotation of Elagolix
Due to the rotation energy barrier of the τ 5 chemical bond (indicated by the red arrow in Figure 2) in different conformational conditions, the mutual transformation between the two conformations is inhibited in the case of Elagolix. Especially under different solvent conditions, there are differences in the rotational energy barriers of chemical bonds, which can result in different peak types and splitting of Elagolix.
Therefore, if we wish to study different hindered isomers separately, alcohol solvents can be used. Under these conditions, hindered isomers have a high energy barrier and can be stably separated (according to literature reports, the conversion time of the two hindered isomers of Elagolix is about 17 hours). On the contrary, if you want to avoid interference from hindered isomers, you can use non proton solvents to reduce the rotational energy barrier, so that two hindered isomers can be quickly converted and only one peak can be displayed in the chromatographic results.
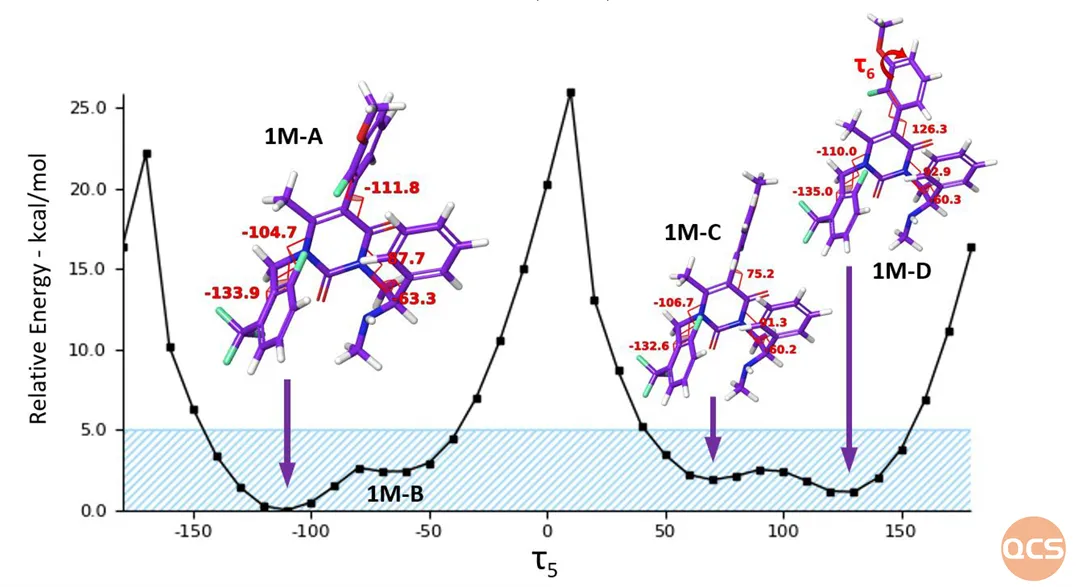
Figure 3: Schematic diagram of the energy barrier for the rotation of the chemical bond of Elagolix τ 5
Data source: Molecules 2023, 28, 3861
The QCS Standard Material R&D Center, in collaboration with Guangzhou R&D Innovation, has established a joint laboratory for chiral standard impurities. The joint laboratory combines the strengths of both parties to conduct comprehensive research on the chiral impurities and their hindered isomers of Elagolix. Using the research and development ENANTIOPAKR ® Y5 chromatographic column was used to study the enantiomers of Elagolix and Elagolix under the condition of ethanol as the eluent.It can be seen that due to the presence of enantiomers, chiral pure Elagolix raw materials and enantiomers exhibit double peaks, and the four peaks do not coincide with each other. This is due to the temporary axial chirality generated by the enantiomers. If the enantiomers in the above chromatographic results are separated, they will also transform into each other after being left for a period of time, which is consistent with the literature (Molecules 2023, 28, 3861.).
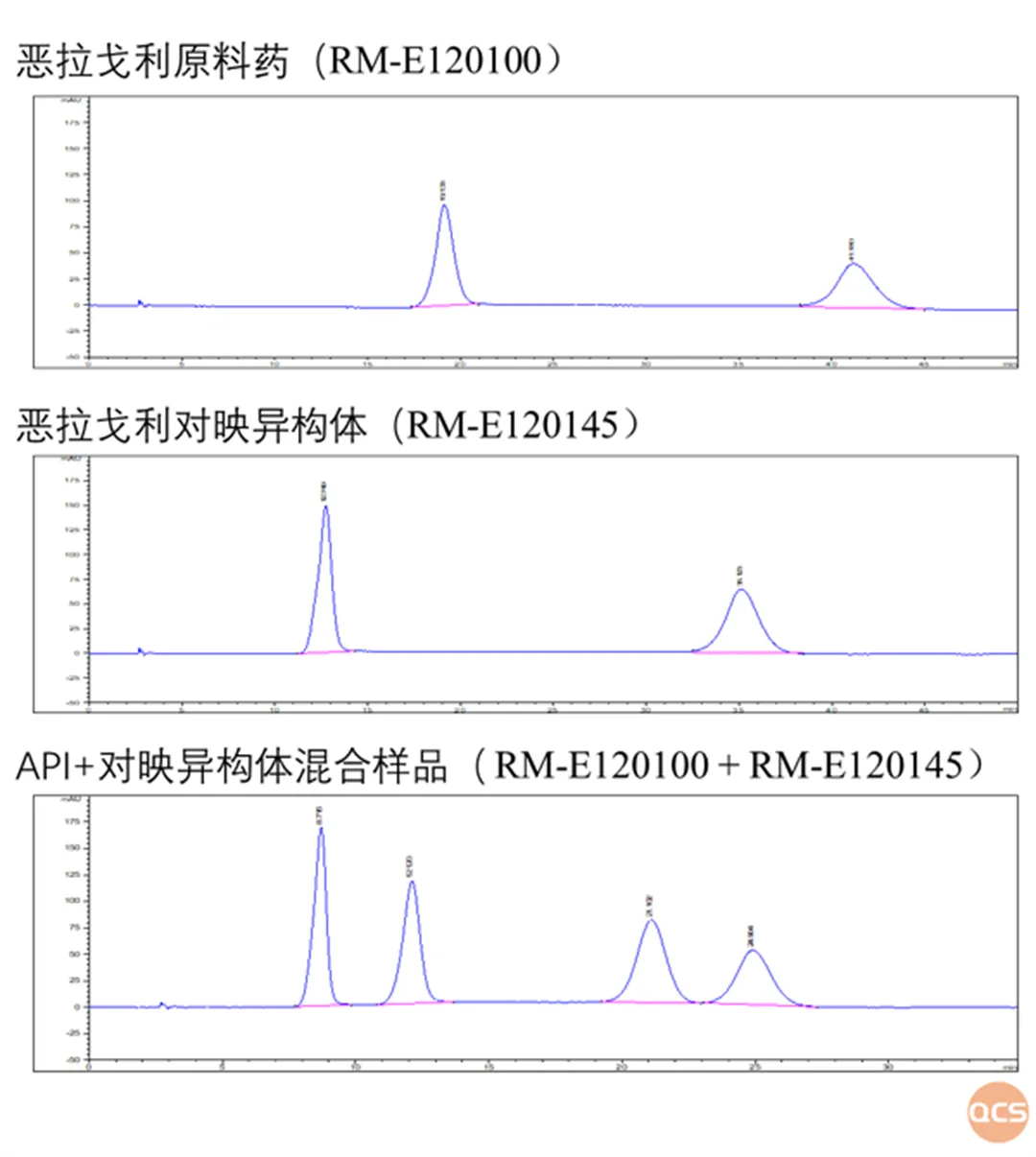
Figure 4: Chiral test results of Elagolix and its enantiomers
Data source: Chiral standard impurity joint laboratory
The skeletal structure of hindered isomers is widely present in natural products, pharmaceutical active molecules, and agricultural chemicals, which will bring new "chirality", opportunities, and challenges. For drug development, hindered enantiomer chirality is a potential threat that significantly increases the cost of drug R&D, and drags down drug development plans. Especially in the field of analysis and detection, due to this special unstable chirality, the presence of hindered isomers can bring huge "troubles" to the analysis and testing. Unlike traditional stable chiral centers, hindered isomers may undergo changes through intramolecular dynamic transformations, which means that the state of hindered isomers is time-dependent, and the half-life of different hindered isomers can fluctuate significantly between minutes and years.
Although the current regulatory authorities do not have direct guidelines or recommendations on the chirality of enantiomers, with the deepening of drug research, enantiomers have become increasingly common and important in the pharmaceutical industry. This article hopes to provide more basic knowledge of enantiomers through a small case study of the QCS standard product research center in the study of Elagolix, hoping to give everyone some help.

Last week, in the article "Research Sharing on Impurities Related to Dysmenorrhea Medications - Elagolix", we mentioned that in the study of the raw materials and enantiomers of Elagolix, we found that there were double peaks in the liquid chromatography results, but the actual purity was actually very high. I think many friends have encountered this problem. Especially in the process of structural characterization, the colleagues of the Elagolix project must have been confused about why high-purity products always have numerous peaks when analyzed by nuclear magnetic resonance spectroscopy, and why the spectra of DMSO-d6, CD3OD, and D2O differ greatly, sometimes even showing two peaks on the high-performance liquid phase and sometimes showing one peak. Although for raw material pharmaceutical products, due to the abundance of standard information, all issues can be briefly addressed with the phrase 'consistent data standards', the research on related substances will bring great challenges
Due to the lack of comparable standard data, the structural characterization and qualitative and quantitative analysis of products require more self completion. For the molecules related to the Elagolix project, although multiple peaks and complex peaks appear in chromatography or nuclear magnetic resonance spectra, this does not mean that there is a problem with product quality. On the contrary, these results are determined by the chemical properties of the compound itself. For example, the special chromatographic results of Elagolix related substances are caused by the problem of hindered isomers. This article will take you to a detailed understanding of what hindered isomers are and what phenomena they may appear in chromatographic and other detection results.

Figure 1: NMR spectra of Elagolix in different solvents under the influence of hindered isomers
Refractive isomers, also known as steric hindrance active isomers, are a class of optically active isomers containing chiral axes. Unlike most chiral compounds formed by asymmetric atoms, optically active isomers containing chiral axes do not necessarily require chemical conditions to convert to each other. Their molecules may form chemical equilibrium when heated to a certain energy. Compared to conformational isomers, hindered isomers must be relatively stable. According to Oki's definition, the half-life of the transformation between hindered isomers at a certain temperature should be greater than 1000 seconds (source: Oki, M; Topics in Stereochemistry 1983, 1)。
The hindered rotation isomer is different from classical new chemical entities, it is just two different conformations produced by the hindrance of chemical bond rotation in one molecule. Due to the fact that two isomers can be converted into each other but have a certain energy barrier, some hindered isomers can be separated by chromatography. Therefore, in chiral HPLC, there will be a double peak (as shown in Figure 2) in the hindered enantiomer of Elagolix.

Figure 2: Schematic diagram of the structural formula and τ 5 chemical bond rotation of Elagolix
Due to the rotation energy barrier of the τ 5 chemical bond (indicated by the red arrow in Figure 2) in different conformational conditions, the mutual transformation between the two conformations is inhibited in the case of Elagolix. Especially under different solvent conditions, there are differences in the rotational energy barriers of chemical bonds, which can result in different peak types and splitting of Elagolix.
Therefore, if we wish to study different hindered isomers separately, alcohol solvents can be used. Under these conditions, hindered isomers have a high energy barrier and can be stably separated (according to literature reports, the conversion time of the two hindered isomers of Elagolix is about 17 hours). On the contrary, if you want to avoid interference from hindered isomers, you can use non proton solvents to reduce the rotational energy barrier, so that two hindered isomers can be quickly converted and only one peak can be displayed in the chromatographic results.

Figure 3: Schematic diagram of the energy barrier for the rotation of the chemical bond of Elagolix τ 5
Data source: Molecules 2023, 28, 3861
The QCS Standard Material R&D Center, in collaboration with Guangzhou R&D Innovation, has established a joint laboratory for chiral standard impurities. The joint laboratory combines the strengths of both parties to conduct comprehensive research on the chiral impurities and their hindered isomers of Elagolix. Using the research and development ENANTIOPAKR ® Y5 chromatographic column was used to study the enantiomers of Elagolix and Elagolix under the condition of ethanol as the eluent.It can be seen that due to the presence of enantiomers, chiral pure Elagolix raw materials and enantiomers exhibit double peaks, and the four peaks do not coincide with each other. This is due to the temporary axial chirality generated by the enantiomers. If the enantiomers in the above chromatographic results are separated, they will also transform into each other after being left for a period of time, which is consistent with the literature (Molecules 2023, 28, 3861.).

Figure 4: Chiral test results of Elagolix and its enantiomers
Data source: Chiral standard impurity joint laboratory
The skeletal structure of hindered isomers is widely present in natural products, pharmaceutical active molecules, and agricultural chemicals, which will bring new "chirality", opportunities, and challenges. For drug development, hindered enantiomer chirality is a potential threat that significantly increases the cost of drug R&D, and drags down drug development plans. Especially in the field of analysis and detection, due to this special unstable chirality, the presence of hindered isomers can bring huge "troubles" to the analysis and testing. Unlike traditional stable chiral centers, hindered isomers may undergo changes through intramolecular dynamic transformations, which means that the state of hindered isomers is time-dependent, and the half-life of different hindered isomers can fluctuate significantly between minutes and years.
Although the current regulatory authorities do not have direct guidelines or recommendations on the chirality of enantiomers, with the deepening of drug research, enantiomers have become increasingly common and important in the pharmaceutical industry. This article hopes to provide more basic knowledge of enantiomers through a small case study of the QCS standard product research center in the study of Elagolix, hoping to give everyone some help.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号