Time:2023-08-11
Liquid chromatography is a commonly used technique, and high-performance liquid chromatography is widely used in the field of analysis and detection. However, most common liquid chromatography systems are analytical systems, using chromatography columns with inner diameters of 2.1 and 4.6 mm, except these, there are also some big guys that may not be familiar to everyone. This article introduces the semi preparative chromatography used in the QCS standard substance R&D center's separation platform, especially the loading and optimization of large-sized chromatography columns.
From a 4.6mm analytical chromatography column to a 100mm experimental preparative chromatography, there are significant differences in both equipment specifications and experimental purposes. Each step is a huge change, and preparative chromatography is different from common analytical chromatography in the laboratory. The experimental requirements range from analytical measurement to preparation separation as the goal. The injection volume has been increased from the ug level of analytical chromatography to the level of 100 grams per needle, and the flow rate has even increased from 1ml/min to 1L/min. The focus of the experiment has also shifted from ensuring good analysis and testing data conversion to efficiently and quickly obtaining the target components. Therefore, efficient and fast separation to obtain as many target components as possible has become the key to preparative chromatography.
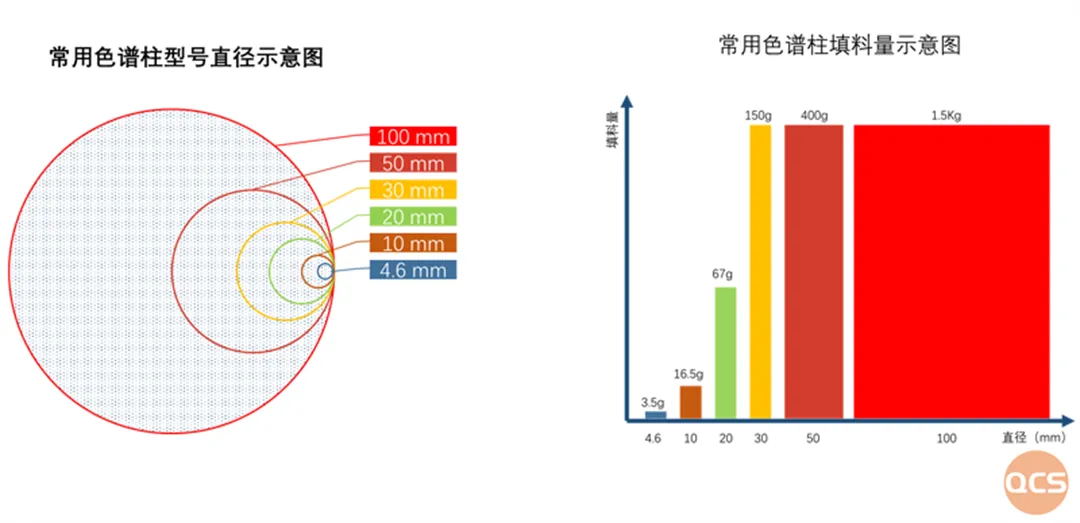
Figure 1: Comparison of common chromatographic column models
Production: QCS Standard Material R&D Center
As the "heart" of the chromatographic system, chromatographic fillers is a prerequisite for achieving the separation of target components. Proper chromatographic fillers and excellent chromatographic columns are the basis for completing preparation and separation. Due to the large column volume in preparative and semi preparative chromatography, the fillers used can be in the kilogram range, the cost of fillers occupies a large part of the preparation and separation work. In order to achieve efficient use of chromatographic fillers, the chromatographic columns used in the preparation of semi preparation services need to be regenerated and refilled. The independent production and loading capacity of chromatographic columns are the determining factors for the separation efficiency and cost of preparation.
The selection and loading of chromatographic fillers is a high-tech task, such as how to select suitable basic fillers, how to make loose fillers into dense and uniform column beds or steel chromatographic columns, and how to regenerate and recover chromatographic fillers, all of which are huge challenges. This article shares with you the experience accumulated by the QCS Standard Material R&D Center in establishing chromatographic systems, especially in converting chromatographic fillers into excellent chromatographic columns/systems. The QCS R&D center uses parallel screening to quickly develop the optimal process for chromatographic column loading for each new type of fillers, in order to maximize the efficiency of chromatographic fillers.
With decades of development in chromatographic fillers, the commonly used types of chromatographic fillers can be divided into three categories based on the matrix: polymer fillers, silica gel matrix fillers, and other inorganic fillers. The fillers used in reverse phase chromatography is often based on silica gel, with a surface bonded phase containing functional groups with relatively weak polarity. Common reverse phase fillers include C18 (ODS), C8 (MOS), C4 (Butyl), C6H5 (Phenyl), etc. The most commonly used chromatographic packing material in the QCS standard substance R&D center is also silica gel matrix. However, due to changes in physical and chemical properties, different surface modified fillers has completely different requirements and performances in the establishment process of chromatographic systems.
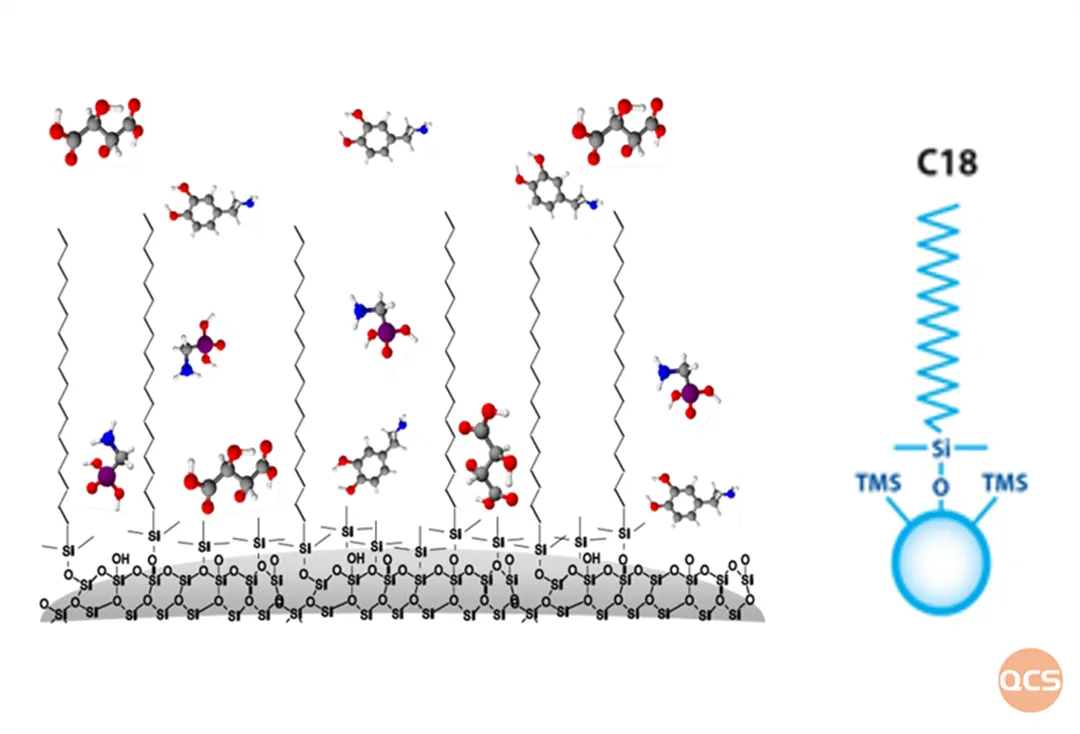
Figure 2: Schematic diagram of C18 reverse phase fillers
Filling loose fillers particles into a compact and uniform chromatographic system is an important task in preparation and separation. Any defects during this process will cause a decrease in chromatographic separation efficiency, such as peak pre extension, tailing, splitting, etc. The construction of typical chromatographic systems usually uses wet homogenization method for filling. The initial fillers particles are dispersed into a suspension in an appropriate solvent, and the liquid is filtered out under pressure conditions to compact the solid fillers particles into a dense chromatographic column on the column bed. This is the general production process of chromatographic columns.
However, due to the different surface modification structures during this process, different types of fillers will form different polymerization states in different solvents. Obtaining a well dispersed and uniform system is the key to establishing a good chromatographic system. Taking the most commonly used C18 as an example, due to the free fatty hydrocarbons on the surface of the modified silicon spheres, the surface alkanes tend to interact with organic solvents. However, the silicon oxygen atoms remaining on the surface of the filler with the participation of silicon spheres are repelled by organic solvents. Therefore, choosing the appropriate solvent system to maintain a subtle balance between the two effects will effectively help the filler particles form a uniformly dispersed system.
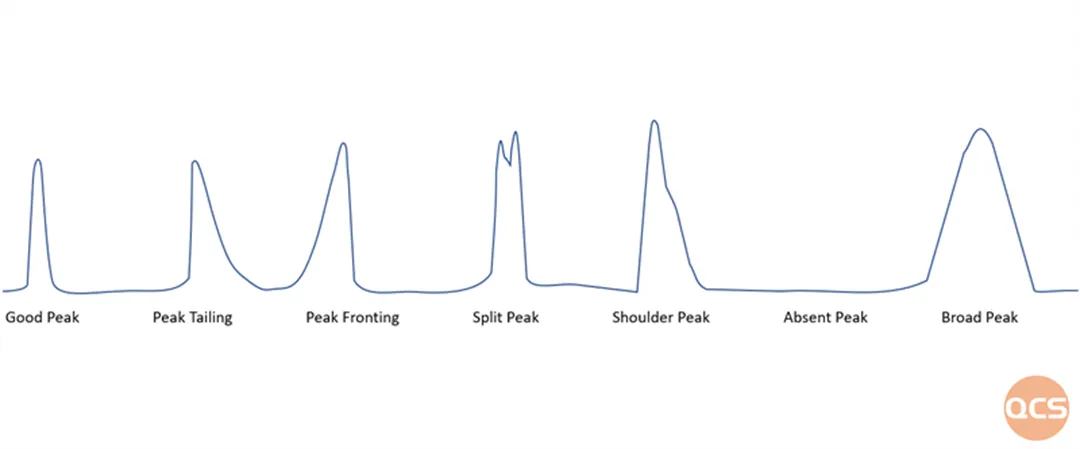
Figure 3: Common chromatographic system peak issues
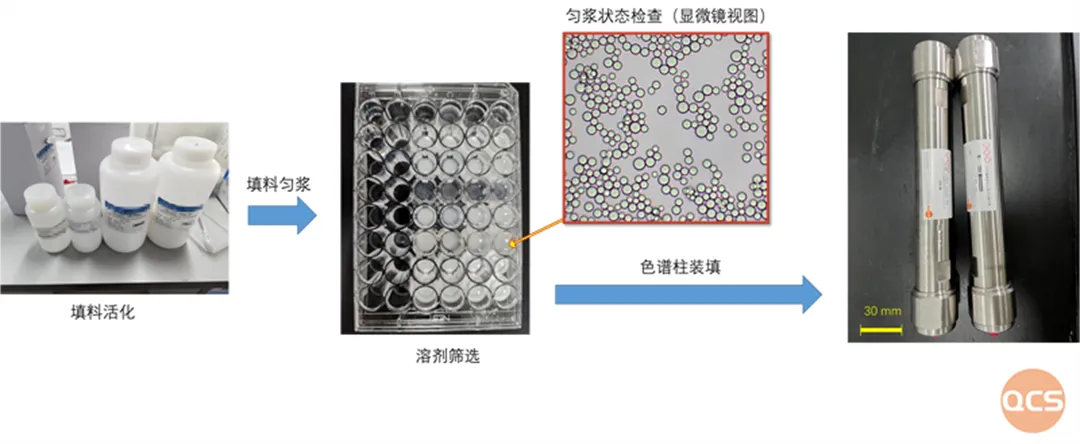
Our center has established different solvent systems, which can complete the construction of various chromatographic systems such as C18, C8, amino, phenyl, hydrophilic fillers, etc. Through the chromatographic production system of QCS standard material R&D center, in-depth research on different fillers is carried out. The center's preparation and separation laboratory has a preparation type chromatographic screening library for different fillers to achieve rapid screening and sample separation of common fillers. For example, under the ETP3 solvent system explored by the QCS standard substance R&D center, multiple fillers exhibited excellent dispersibility (see video 1, filler particle size 10um). The chromatography system/column constructed under this condition showed extremely excellent chromatographic separation performance, and its sample separation ability was close to that of corresponding products from well-known foreign brands. It can efficiently and conveniently provide customers with high-quality preparation and separation services.
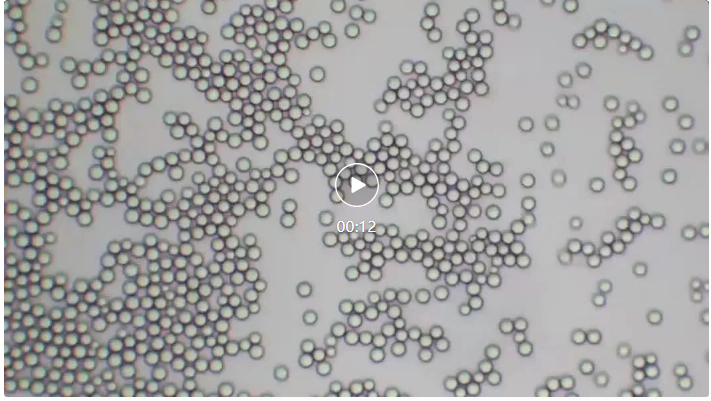
Video 1: Solvent dispersion effect of preparation type 10um silicone matrix filler
Shooting: QCS Standard Material R&D Center
Magnification factor: 200x, solvent: D-type
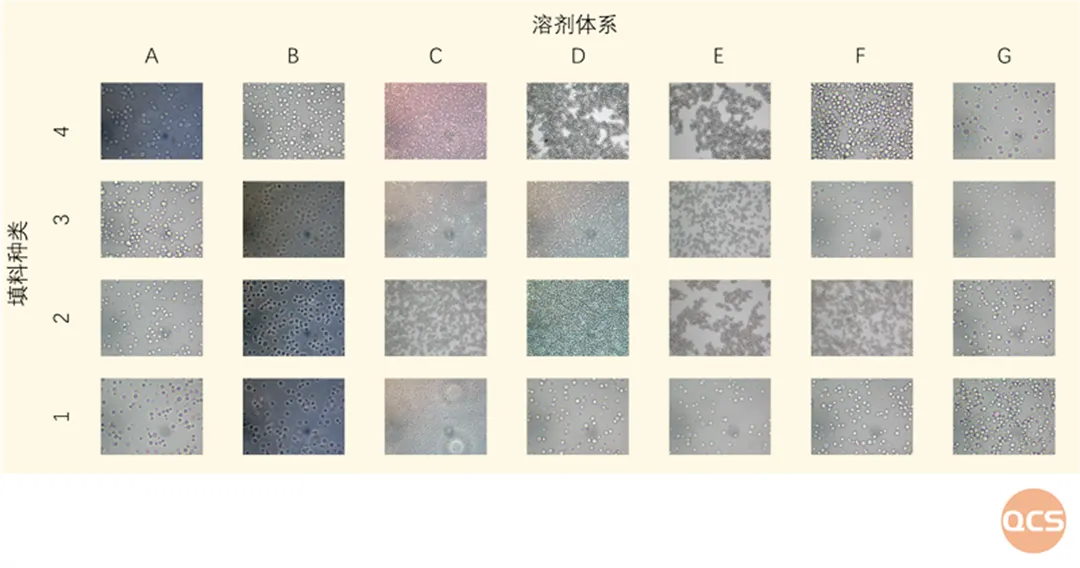
Figure 4: Chromatographic packing/solvent screening case
Data source: QCS Standard Material R&D Center
Relying on the full chain of technical reserves and R&D capabilities, the QCS Standard Material R&D Center Preparation and Separation Laboratory is committed to solving the needs of pharmaceutical customers for impurity separation and purification. The laboratory provides full process technical support from filler screening, chromatographic loading and assembly, and engineering applications. Through our technological accumulation, we strive for excellence in every detail, providing customers with superior services with outstanding technical strength, higher standards, and higher efficiency.

Liquid chromatography is a commonly used technique, and high-performance liquid chromatography is widely used in the field of analysis and detection. However, most common liquid chromatography systems are analytical systems, using chromatography columns with inner diameters of 2.1 and 4.6 mm, except these, there are also some big guys that may not be familiar to everyone. This article introduces the semi preparative chromatography used in the QCS standard substance R&D center's separation platform, especially the loading and optimization of large-sized chromatography columns.
From a 4.6mm analytical chromatography column to a 100mm experimental preparative chromatography, there are significant differences in both equipment specifications and experimental purposes. Each step is a huge change, and preparative chromatography is different from common analytical chromatography in the laboratory. The experimental requirements range from analytical measurement to preparation separation as the goal. The injection volume has been increased from the ug level of analytical chromatography to the level of 100 grams per needle, and the flow rate has even increased from 1ml/min to 1L/min. The focus of the experiment has also shifted from ensuring good analysis and testing data conversion to efficiently and quickly obtaining the target components. Therefore, efficient and fast separation to obtain as many target components as possible has become the key to preparative chromatography.

Figure 1: Comparison of common chromatographic column models
Production: QCS Standard Material R&D Center
As the "heart" of the chromatographic system, chromatographic fillers is a prerequisite for achieving the separation of target components. Proper chromatographic fillers and excellent chromatographic columns are the basis for completing preparation and separation. Due to the large column volume in preparative and semi preparative chromatography, the fillers used can be in the kilogram range, the cost of fillers occupies a large part of the preparation and separation work. In order to achieve efficient use of chromatographic fillers, the chromatographic columns used in the preparation of semi preparation services need to be regenerated and refilled. The independent production and loading capacity of chromatographic columns are the determining factors for the separation efficiency and cost of preparation.
The selection and loading of chromatographic fillers is a high-tech task, such as how to select suitable basic fillers, how to make loose fillers into dense and uniform column beds or steel chromatographic columns, and how to regenerate and recover chromatographic fillers, all of which are huge challenges. This article shares with you the experience accumulated by the QCS Standard Material R&D Center in establishing chromatographic systems, especially in converting chromatographic fillers into excellent chromatographic columns/systems. The QCS R&D center uses parallel screening to quickly develop the optimal process for chromatographic column loading for each new type of fillers, in order to maximize the efficiency of chromatographic fillers.
With decades of development in chromatographic fillers, the commonly used types of chromatographic fillers can be divided into three categories based on the matrix: polymer fillers, silica gel matrix fillers, and other inorganic fillers. The fillers used in reverse phase chromatography is often based on silica gel, with a surface bonded phase containing functional groups with relatively weak polarity. Common reverse phase fillers include C18 (ODS), C8 (MOS), C4 (Butyl), C6H5 (Phenyl), etc. The most commonly used chromatographic packing material in the QCS standard substance R&D center is also silica gel matrix. However, due to changes in physical and chemical properties, different surface modified fillers has completely different requirements and performances in the establishment process of chromatographic systems.

Figure 2: Schematic diagram of C18 reverse phase fillers
Filling loose fillers particles into a compact and uniform chromatographic system is an important task in preparation and separation. Any defects during this process will cause a decrease in chromatographic separation efficiency, such as peak pre extension, tailing, splitting, etc. The construction of typical chromatographic systems usually uses wet homogenization method for filling. The initial fillers particles are dispersed into a suspension in an appropriate solvent, and the liquid is filtered out under pressure conditions to compact the solid fillers particles into a dense chromatographic column on the column bed. This is the general production process of chromatographic columns.
However, due to the different surface modification structures during this process, different types of fillers will form different polymerization states in different solvents. Obtaining a well dispersed and uniform system is the key to establishing a good chromatographic system. Taking the most commonly used C18 as an example, due to the free fatty hydrocarbons on the surface of the modified silicon spheres, the surface alkanes tend to interact with organic solvents. However, the silicon oxygen atoms remaining on the surface of the filler with the participation of silicon spheres are repelled by organic solvents. Therefore, choosing the appropriate solvent system to maintain a subtle balance between the two effects will effectively help the filler particles form a uniformly dispersed system.

Figure 3: Common chromatographic system peak issues

Our center has established different solvent systems, which can complete the construction of various chromatographic systems such as C18, C8, amino, phenyl, hydrophilic fillers, etc. Through the chromatographic production system of QCS standard material R&D center, in-depth research on different fillers is carried out. The center's preparation and separation laboratory has a preparation type chromatographic screening library for different fillers to achieve rapid screening and sample separation of common fillers. For example, under the ETP3 solvent system explored by the QCS standard substance R&D center, multiple fillers exhibited excellent dispersibility (see video 1, filler particle size 10um). The chromatography system/column constructed under this condition showed extremely excellent chromatographic separation performance, and its sample separation ability was close to that of corresponding products from well-known foreign brands. It can efficiently and conveniently provide customers with high-quality preparation and separation services.

Video 1: Solvent dispersion effect of preparation type 10um silicone matrix filler
Shooting: QCS Standard Material R&D Center
Magnification factor: 200x, solvent: D-type

Figure 4: Chromatographic packing/solvent screening case
Data source: QCS Standard Material R&D Center
Relying on the full chain of technical reserves and R&D capabilities, the QCS Standard Material R&D Center Preparation and Separation Laboratory is committed to solving the needs of pharmaceutical customers for impurity separation and purification. The laboratory provides full process technical support from filler screening, chromatographic loading and assembly, and engineering applications. Through our technological accumulation, we strive for excellence in every detail, providing customers with superior services with outstanding technical strength, higher standards, and higher efficiency.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号