Time:2023-09-14
Introduction: Today, we present a study on impurities related to the widely used drug fodosteine, and we would like to express our gratitude to our clients for supplying the criteria and relevant data for this research.
Fodosteine is primarily indicated for the treatment of bronchial asthma, emphysema, chronic bronchitis, bronchiectasis tuberculosis, and other related conditions. Currently, it is available in the market mainly as tablets, capsules, and oral solutions. According to pharmaceutical intelligence data, most enterprises involved in its declaration are classified under three categories of chemical drugs, with approximately 15 companies having submitted declarations (including approval documents).
For projects related to fodostein, QCS brand offers a diverse range of products, including process magazines and degradation impurities. Currently, the QCS official website lists over 40 impurities associated with Fodostein (please scan the QR code at the end of this article for a comprehensive list). The QCS Standard Material Research and Development Center (hereafter referred to as 'our center') has conducted in-depth studies on several specific fodostein-related impurities—specifically sulfone and sulfoxide impurities discussed in Part 1, and DL-malic acid adduct impurities covered in Part 2—drawing upon public notices regarding relevant quality standards for fodostein raw materials and tablets, as well as literature from JP pharmacopoeia. This paper aims to share our center's pertinent research data with readers. The structural information pertaining to the impurities discussed herein is illustrated in Figure 1.
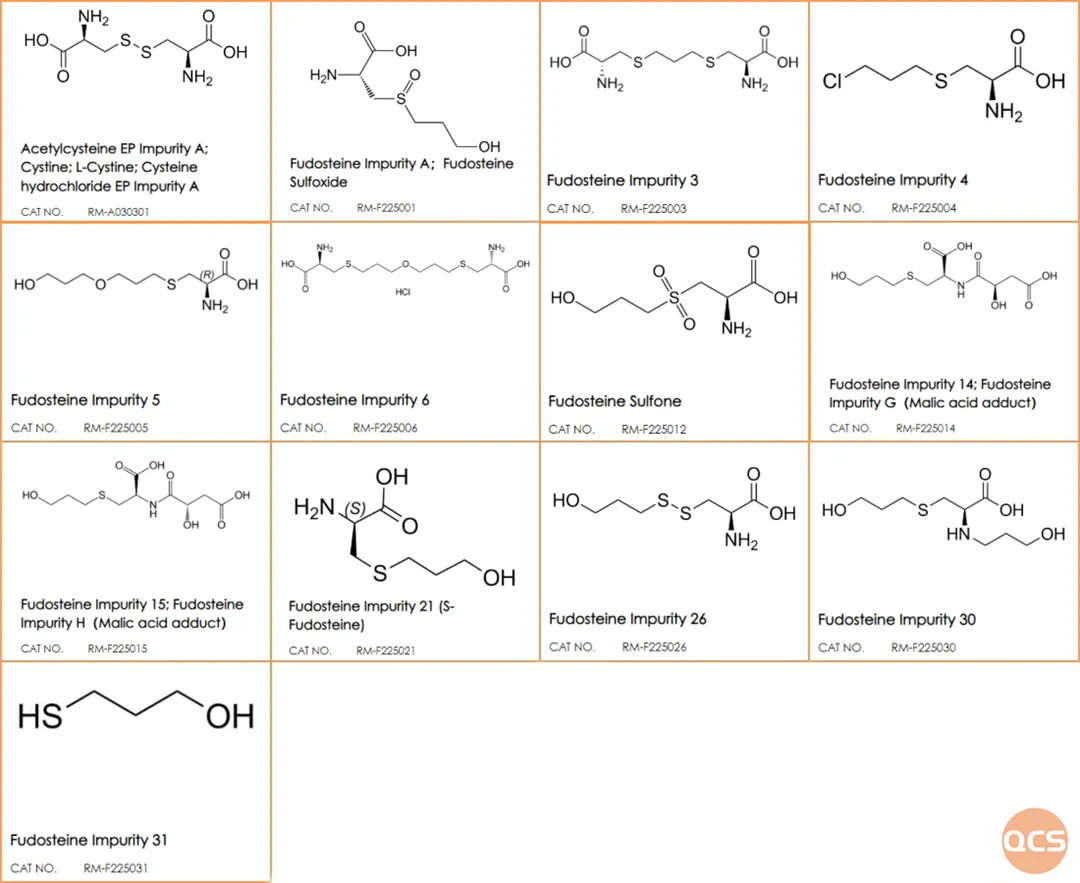
Figure 1: Compilation of impurities examined in the study of Fodosteine
Section One: Investigations into Impurities of Fodosteine Oxide, Sulfone, and Sulfoxide
The public information regarding the quality standards of Fodosteine raw materials and tablets includes four major impurities, each accompanied by a specific detection method. In this study, extensive reference to and application of the relevant detection conditions outlined in the table were employed to investigate the tenfold difference in absorption strength between two oxidation impurities of Fodosteine: Fodosteine sulfoxide impurity (RM-F225001) and Fodosteine sulfoxide impurity (RM-F225012). Our center prepared solutions of these sulfoxide reference products at comparable concentrations. Under identical chromatographic conditions, both products were analyzed using liquid chromatography (refer to Figure 2), and relative correction factors for the products were calculated (see Figure 3). The results are presented below.
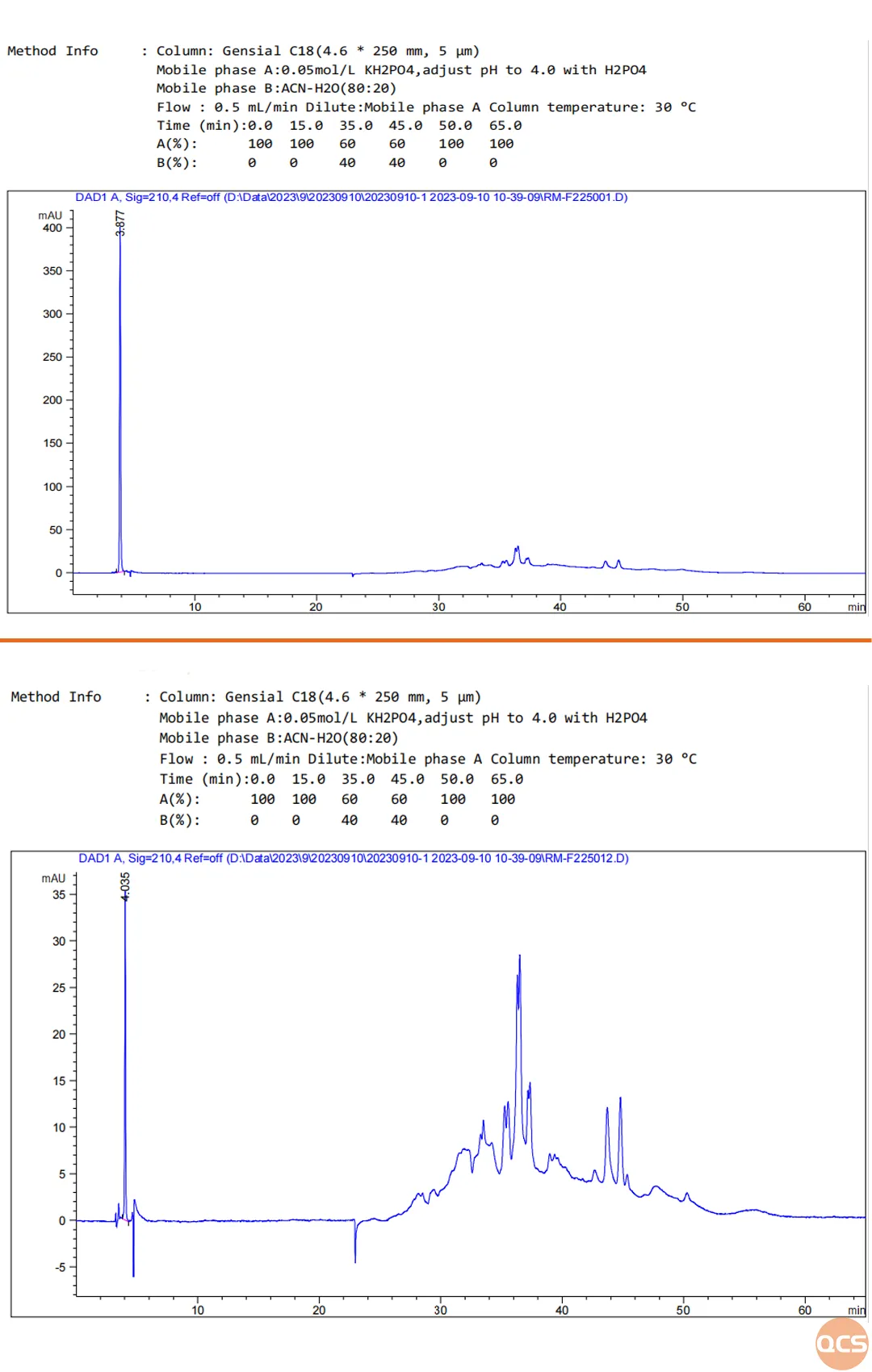
Figure 2: Liquid phase profiles of fordosteine sulfoxide and its sulfoxide counterpart at comparable concentrations with identical sample sizes
As illustrated in Figure 2, under conditions of comparable concentration and equal injection volume (approximately 0.3 mg/mL with a sample size of 10 µL), the absorption intensities of fordosteine sulfoxide and its sulfone impurities at a wavelength of 210 nm exhibit significant differences. By precisely calculating the concentration values and comparing the relative absorption strengths of both products, we can derive their respective correction factors, as presented in Figure 3.
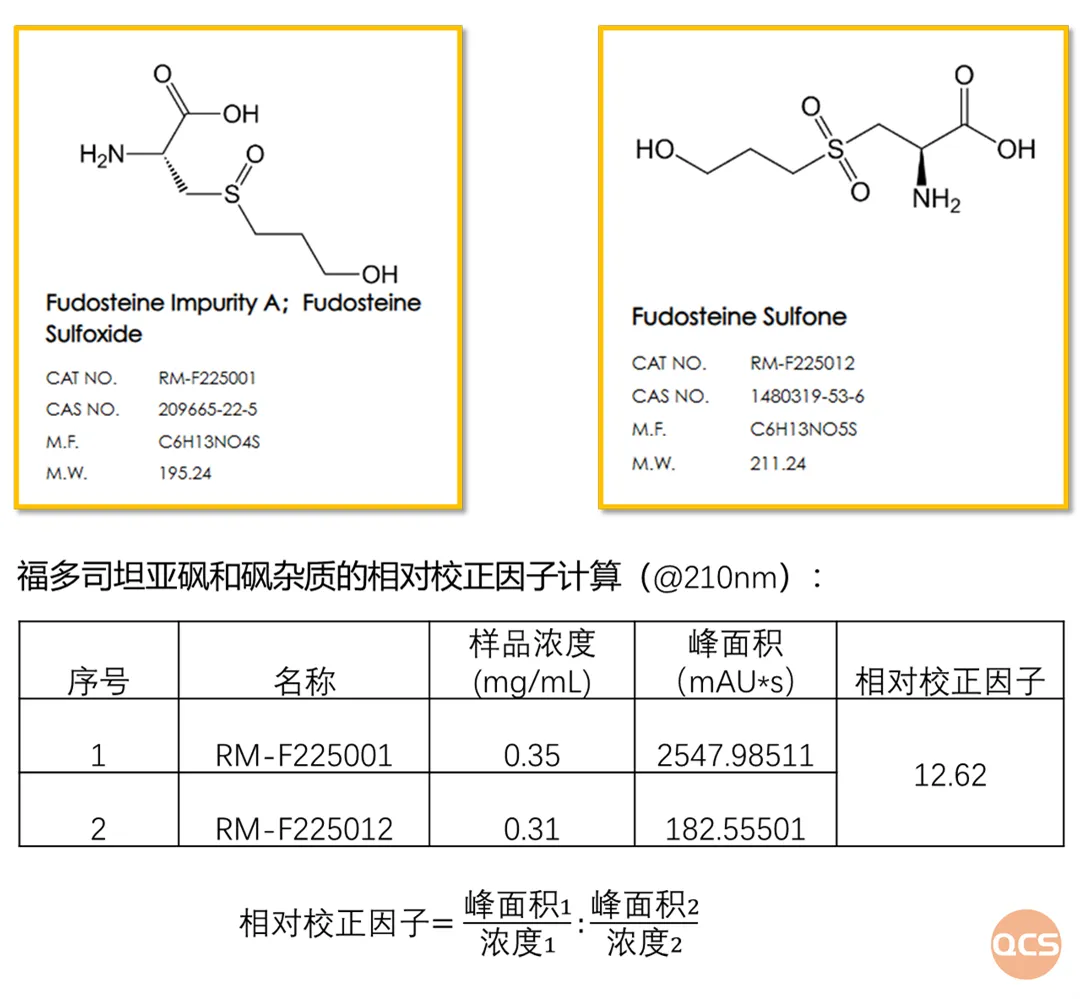
Figure 3: Relative correction factors for fodosteine sulfoxide and its sulfoxide impurity at a wavelength of 210 nm
Based on the relative correction factor for fordosteine sulfoxide and its sulfoxide impurity, the peak area of sulfoxide (RM-F225001) is 12.6 times greater than that of sulfoxide (RM-F225012) at equivalent concentrations. Consequently, during the actual research process, if simultaneous results for both impurity samples are required within the same chromatographic analysis, it is essential to adjust the sample concentration according to research objectives to ensure a relatively balanced signal strength for both impurities. Drawing from extensive practical experience among various clients, employing distinct methodologies to manage these two impurities proves to be more effective.
Section Two: Investigations into the Impurities of Fodosteine DL-Malic Acid Adduct
As the first drug approved for listing in Japan in December 2001, the pertinent information regarding this product disclosed by the Japanese PMDA is essential for pharmaceutical companies to reference during their quality control processes. According to the IF file data from PMDA (FIG. 4), Fodosteine oral liquid comprises various components, including D-sorbitol, DL-malic acid, caramel, sodium benzoate, flavoring agents, vanillin, ethyl vanillin, ethanol, and glycerin. The interactions between these additives and raw materials can lead to the formation of different impurity components; notably, DL-malic acid and fodosteine adduct impurities represent a significant aspect of impurity research.
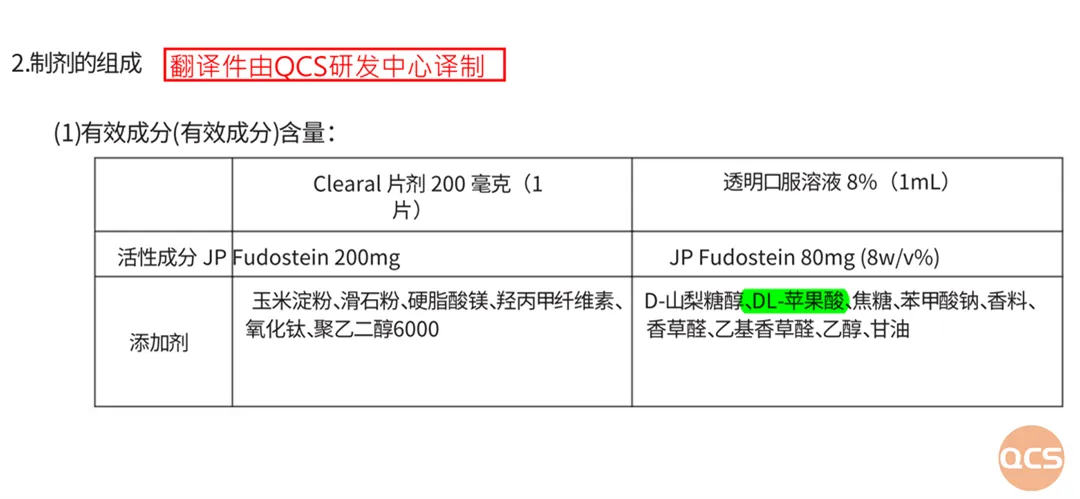
Figure 4: Fodosteine IF File Information
Source: Pharmaceuticals and Medical Devices Agency (PMDA), Japan
Translation: QCS Standard Material Research and Development Center
Our center is equipped to provide impurity samples of D-malic acid and L-malic acid, each combined with fodosteine, for the purposes of this project study. The impurity products (Item No.: RM-F225014, RM-F225015) correspond to decomposition product G and decomposition product H as detailed in the IF file published by PMDA (refer to Figure 5).
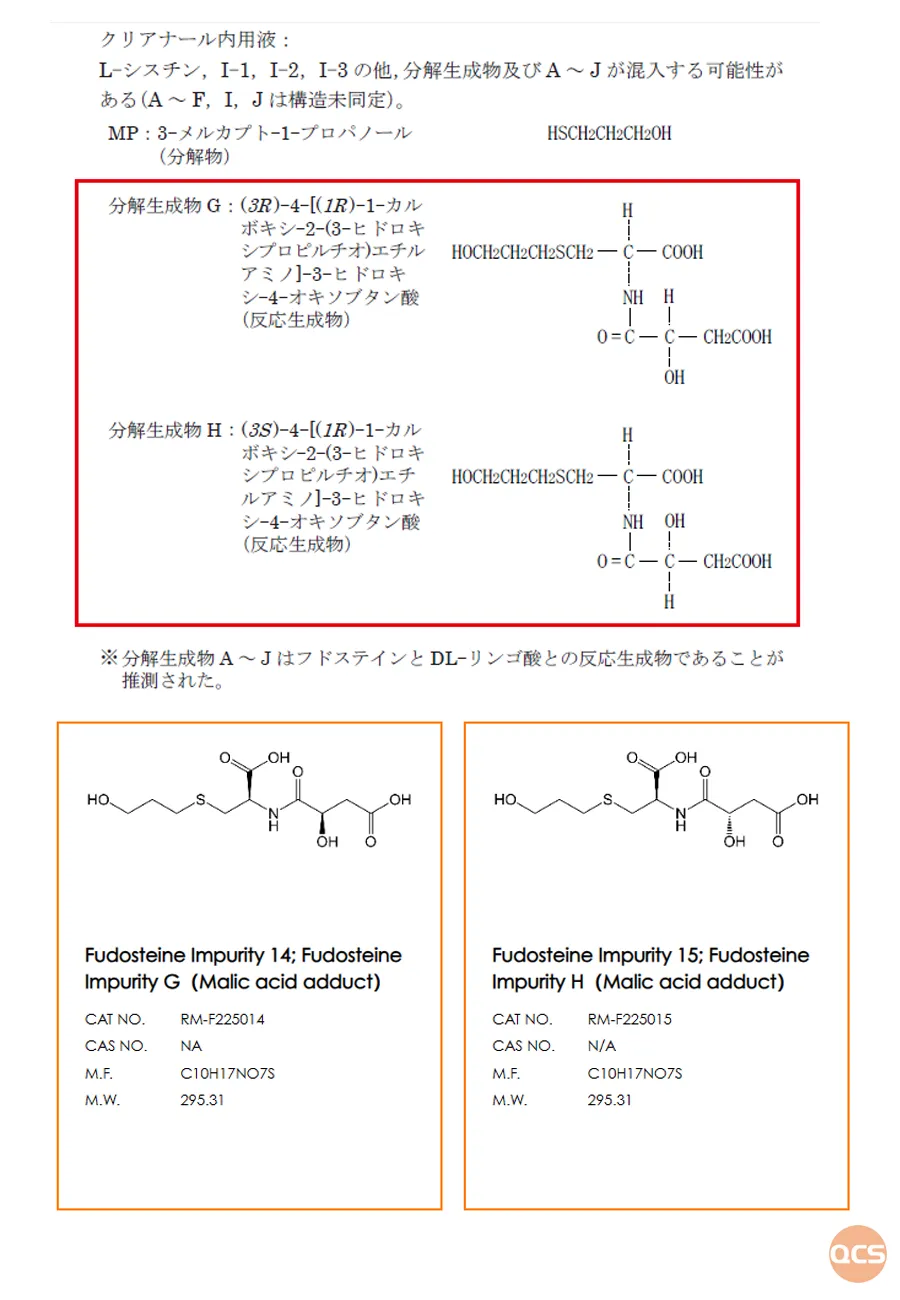
Figure 5: Structural representation of the adduct formed between fodosteine and DL-malic acid
Data source: QCS Standard Materials Research and Development Center
Utilizing the JP method as a reference, our center performed chromatographic analyses on the impurities present in fodosteine raw materials and D/L-malic acid when combined with fodosteine, respectively, confirming that these impurities can be effectively separated. The specific chromatographic data are presented in Figure 6.
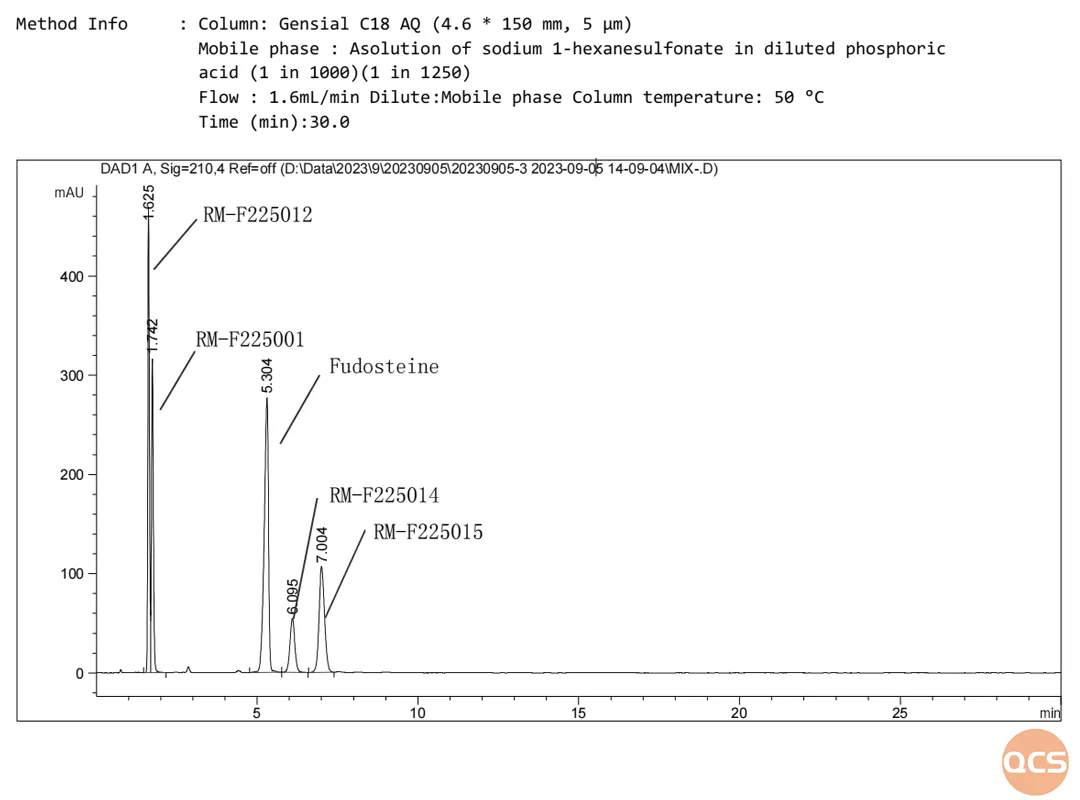
Figure 6: Fodosteine undergoes a reaction with DL-malic acid, resulting in a mixture of degradation products
Data source: QCS Standard Materials Research and Development Center
The chromatographic analysis of the mixed samples (FIG. 6) demonstrates that the degradation products formed from the interaction between fodosteine and DL-malic acid exhibit effective separation, with relative retention time information presented in FIG. 7.
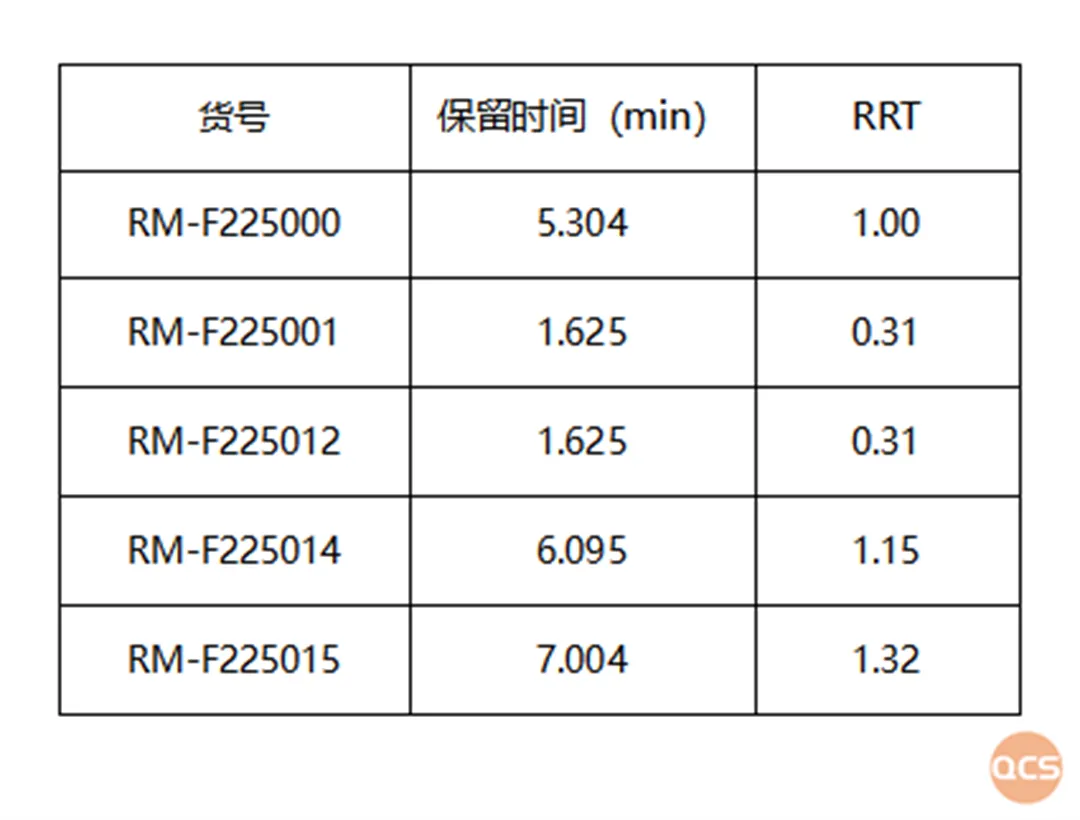
Figure 7: Overview of the mixed injection data pertaining to degradation products generated from the reaction between fodosteine and DL-malic acid
Data source: QCS Standard Materials Research and Development Center
Finally, data regarding the correlation between fodosteine and impurities in DL-malic acid admixtures are presented. The experimental results stem from a study aimed at inducing impurities in DL-malic acid admixtures of fodosteine under high-temperature conditions. In this investigation, researchers conducted a forced degradation process at 60°C. During this process, the relationship between the quantity of DL-malic acid and the concentration of its associated impurities was examined by varying the amount of DL-malic acid added while using pH as an index. The findings indicate that there is a positive correlation between the quantity of DL-malic acid and the levels of target impurities (RM-F225014 & RM-F225015, highlighted in red in the table). As the amount of DL-malic acid increased, so did the concentration of these target impurities. Detailed data are summarized in Figure 8.
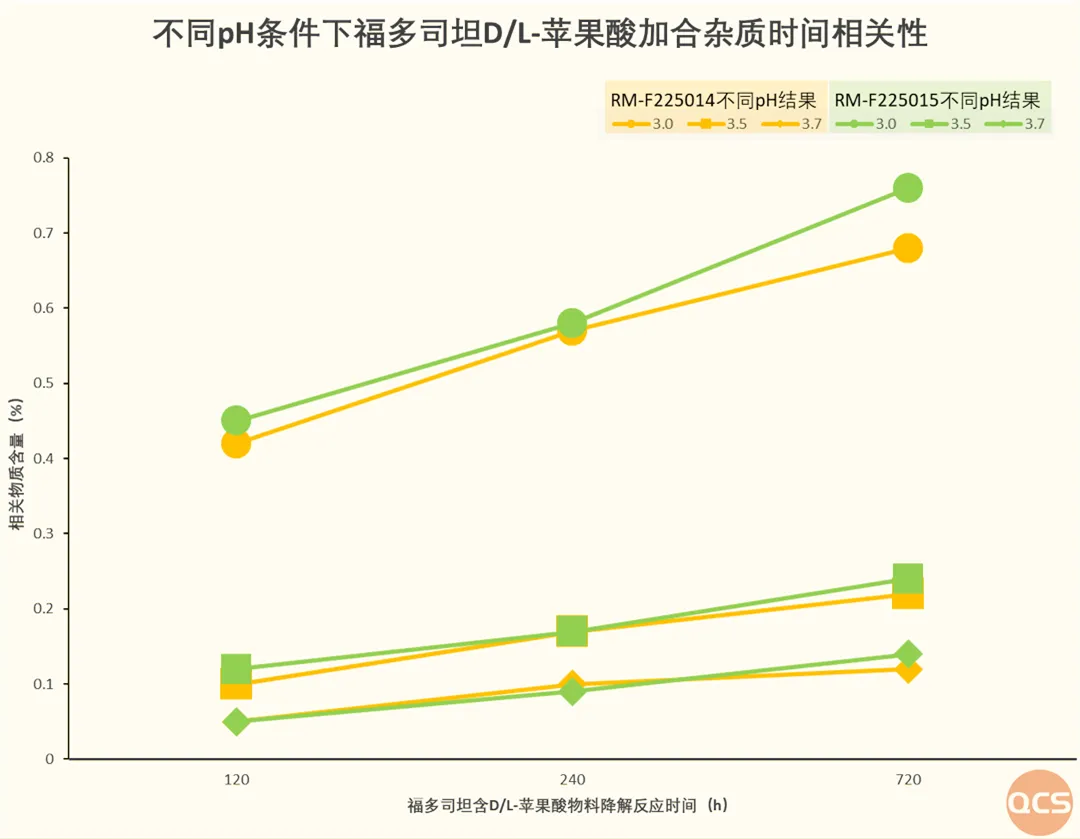
Figure 8: Temporal correlation of fodosteine and DL-malic acid admixtures across varying pH levels
Summary
In this study on the impurities of fodosteine, we primarily present the relative correction factors for sulfone oxide and sulfoxide impurities identified at our center. Additionally, we provide insights into the impurities associated with the fodosteine DL-malate adduct, with the aim of supporting your research endeavors. We would also like to express our gratitude for the comprehensive analysis of malic acid addition products shared by our clients, and we hope that this information will serve as a valuable reference for those developing this particular compound.
For further details on all identified impurities, please long-press the QR code.


Introduction: Today, we present a study on impurities related to the widely used drug fodosteine, and we would like to express our gratitude to our clients for supplying the criteria and relevant data for this research.
Fodosteine is primarily indicated for the treatment of bronchial asthma, emphysema, chronic bronchitis, bronchiectasis tuberculosis, and other related conditions. Currently, it is available in the market mainly as tablets, capsules, and oral solutions. According to pharmaceutical intelligence data, most enterprises involved in its declaration are classified under three categories of chemical drugs, with approximately 15 companies having submitted declarations (including approval documents).
For projects related to fodostein, QCS brand offers a diverse range of products, including process magazines and degradation impurities. Currently, the QCS official website lists over 40 impurities associated with Fodostein (please scan the QR code at the end of this article for a comprehensive list). The QCS Standard Material Research and Development Center (hereafter referred to as 'our center') has conducted in-depth studies on several specific fodostein-related impurities—specifically sulfone and sulfoxide impurities discussed in Part 1, and DL-malic acid adduct impurities covered in Part 2—drawing upon public notices regarding relevant quality standards for fodostein raw materials and tablets, as well as literature from JP pharmacopoeia. This paper aims to share our center's pertinent research data with readers. The structural information pertaining to the impurities discussed herein is illustrated in Figure 1.

Figure 1: Compilation of impurities examined in the study of Fodosteine
Section One: Investigations into Impurities of Fodosteine Oxide, Sulfone, and Sulfoxide
The public information regarding the quality standards of Fodosteine raw materials and tablets includes four major impurities, each accompanied by a specific detection method. In this study, extensive reference to and application of the relevant detection conditions outlined in the table were employed to investigate the tenfold difference in absorption strength between two oxidation impurities of Fodosteine: Fodosteine sulfoxide impurity (RM-F225001) and Fodosteine sulfoxide impurity (RM-F225012). Our center prepared solutions of these sulfoxide reference products at comparable concentrations. Under identical chromatographic conditions, both products were analyzed using liquid chromatography (refer to Figure 2), and relative correction factors for the products were calculated (see Figure 3). The results are presented below.

Figure 2: Liquid phase profiles of fordosteine sulfoxide and its sulfoxide counterpart at comparable concentrations with identical sample sizes
As illustrated in Figure 2, under conditions of comparable concentration and equal injection volume (approximately 0.3 mg/mL with a sample size of 10 µL), the absorption intensities of fordosteine sulfoxide and its sulfone impurities at a wavelength of 210 nm exhibit significant differences. By precisely calculating the concentration values and comparing the relative absorption strengths of both products, we can derive their respective correction factors, as presented in Figure 3.

Figure 3: Relative correction factors for fodosteine sulfoxide and its sulfoxide impurity at a wavelength of 210 nm
Based on the relative correction factor for fordosteine sulfoxide and its sulfoxide impurity, the peak area of sulfoxide (RM-F225001) is 12.6 times greater than that of sulfoxide (RM-F225012) at equivalent concentrations. Consequently, during the actual research process, if simultaneous results for both impurity samples are required within the same chromatographic analysis, it is essential to adjust the sample concentration according to research objectives to ensure a relatively balanced signal strength for both impurities. Drawing from extensive practical experience among various clients, employing distinct methodologies to manage these two impurities proves to be more effective.
Section Two: Investigations into the Impurities of Fodosteine DL-Malic Acid Adduct
As the first drug approved for listing in Japan in December 2001, the pertinent information regarding this product disclosed by the Japanese PMDA is essential for pharmaceutical companies to reference during their quality control processes. According to the IF file data from PMDA (FIG. 4), Fodosteine oral liquid comprises various components, including D-sorbitol, DL-malic acid, caramel, sodium benzoate, flavoring agents, vanillin, ethyl vanillin, ethanol, and glycerin. The interactions between these additives and raw materials can lead to the formation of different impurity components; notably, DL-malic acid and fodosteine adduct impurities represent a significant aspect of impurity research.

Figure 4: Fodosteine IF File Information
Source: Pharmaceuticals and Medical Devices Agency (PMDA), Japan
Translation: QCS Standard Material Research and Development Center
Our center is equipped to provide impurity samples of D-malic acid and L-malic acid, each combined with fodosteine, for the purposes of this project study. The impurity products (Item No.: RM-F225014, RM-F225015) correspond to decomposition product G and decomposition product H as detailed in the IF file published by PMDA (refer to Figure 5).

Figure 5: Structural representation of the adduct formed between fodosteine and DL-malic acid
Data source: QCS Standard Materials Research and Development Center
Utilizing the JP method as a reference, our center performed chromatographic analyses on the impurities present in fodosteine raw materials and D/L-malic acid when combined with fodosteine, respectively, confirming that these impurities can be effectively separated. The specific chromatographic data are presented in Figure 6.

Figure 6: Fodosteine undergoes a reaction with DL-malic acid, resulting in a mixture of degradation products
Data source: QCS Standard Materials Research and Development Center
The chromatographic analysis of the mixed samples (FIG. 6) demonstrates that the degradation products formed from the interaction between fodosteine and DL-malic acid exhibit effective separation, with relative retention time information presented in FIG. 7.

Figure 7: Overview of the mixed injection data pertaining to degradation products generated from the reaction between fodosteine and DL-malic acid
Data source: QCS Standard Materials Research and Development Center
Finally, data regarding the correlation between fodosteine and impurities in DL-malic acid admixtures are presented. The experimental results stem from a study aimed at inducing impurities in DL-malic acid admixtures of fodosteine under high-temperature conditions. In this investigation, researchers conducted a forced degradation process at 60°C. During this process, the relationship between the quantity of DL-malic acid and the concentration of its associated impurities was examined by varying the amount of DL-malic acid added while using pH as an index. The findings indicate that there is a positive correlation between the quantity of DL-malic acid and the levels of target impurities (RM-F225014 & RM-F225015, highlighted in red in the table). As the amount of DL-malic acid increased, so did the concentration of these target impurities. Detailed data are summarized in Figure 8.

Figure 8: Temporal correlation of fodosteine and DL-malic acid admixtures across varying pH levels
Summary
In this study on the impurities of fodosteine, we primarily present the relative correction factors for sulfone oxide and sulfoxide impurities identified at our center. Additionally, we provide insights into the impurities associated with the fodosteine DL-malate adduct, with the aim of supporting your research endeavors. We would also like to express our gratitude for the comprehensive analysis of malic acid addition products shared by our clients, and we hope that this information will serve as a valuable reference for those developing this particular compound.
For further details on all identified impurities, please long-press the QR code.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号