Time:2023-07-27
Introduction: Today, we present an impurity study on a relatively niche pharmaceutical compound - Elagolix. This particular variant is being discussed primarily due to the client's observation of anomalous results in the detection of the corresponding isomer of Elagolix. We will elaborate on the irregularities encountered.
Elagolix (trade name: Orilissa) is an oral gonadotropin-releasing hormone (GnRH) antagonist that received approval from the US FDA in 2018 for the management of moderate to severe pain associated with endometriosis, specifically dysmenorrhea.
Currently, over 90 Elagolix impurities have been cataloged on the official QCS website (please scan the QR code at the end of this article to access the complete list of impurities). Our center has performed a liquid chromatographic characterization study on these impurities in accordance with our company's internal control standards for raw materials. The specific chromatographic methodology is illustrated in FIG. 1, while the results from mixed injection liquid chromatography are presented in FIG. 2.
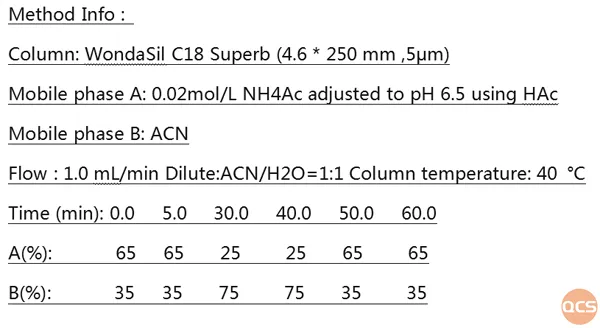
Figure 1: regulation of chromatographic conditions internally
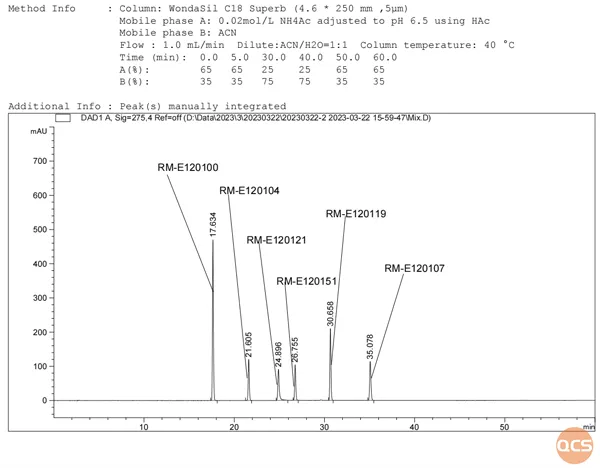
Figure 2: Mixed injection Figure 1-275nm
Figure 3 illustrates the detection of distinct wavelengths utilizing a uniform methodology for mixed samples:
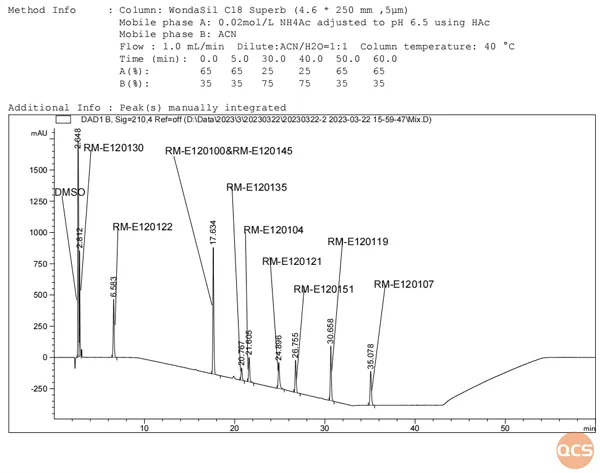
Figure 3: Mixed injection Figure 2-210nm
As illustrated in Figures 2 and 3, nearly all impurities exhibit a significant degree of separation from API (RM-E120100). Figure 3 demonstrates that Elagolix (RM-E120100) and S-Elagolix (RM-E120145) overlap; subsequently, we combined the samples using the same methodology, with the corresponding isomers presented in Figure 4 and their structural formulas depicted in Figure 5.
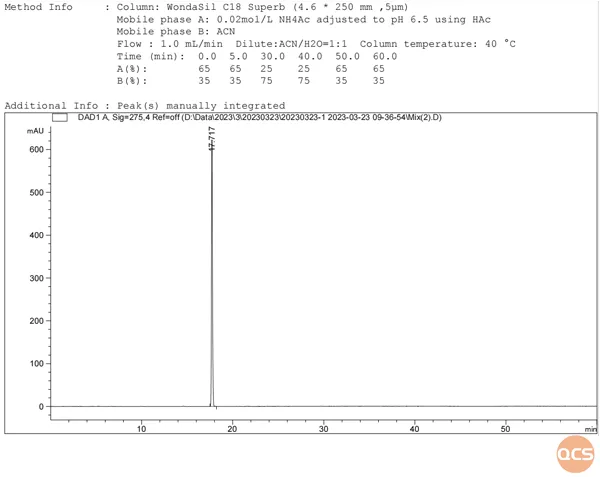
Figure 4: schematic representation of the mixed injection process for Elagolix and its corresponding isomer, S-Elagolix
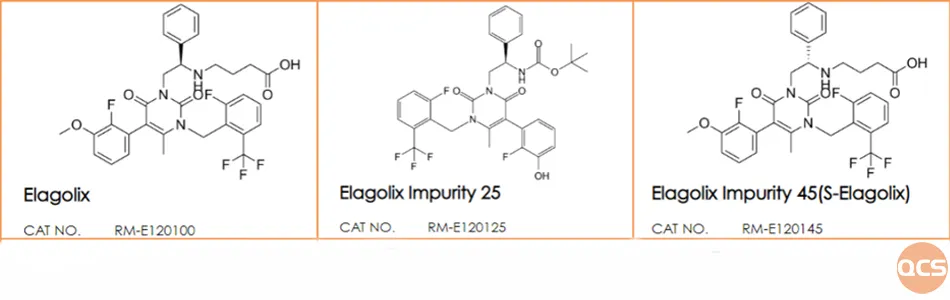
Figure 5: structural formula of Elagolix and its corresponding isomer,S-Elagolix
As illustrated in FIG. 4, Elagolix and S-Elagolix exhibit complete congruence within the inverted phase system. Subsequently, we re-evaluated the optical rotation data for both Elagolix and S-Elagolix, with the specific results presented in FIG. 6.
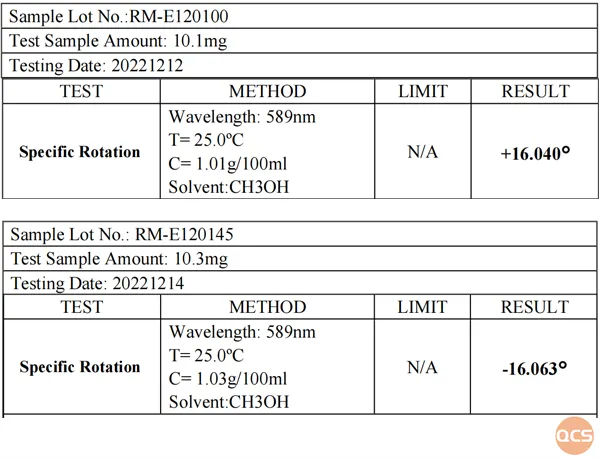
Figure 6: optical rotation data for Elagolix (RM-E120100) and S-Elagolix (RM-E120145)
Astute readers may question whether structural confirmation and optical rotation data alone are sufficient to ascertain the corresponding isomers. The conventional approach involves analyzing these isomers through chiral analytical methods. Consequently, our company has developed advanced chiral analysis techniques and successfully separated and tested the products through Chiral Standard Impurity Laboratory of QCS and R&Chiral.
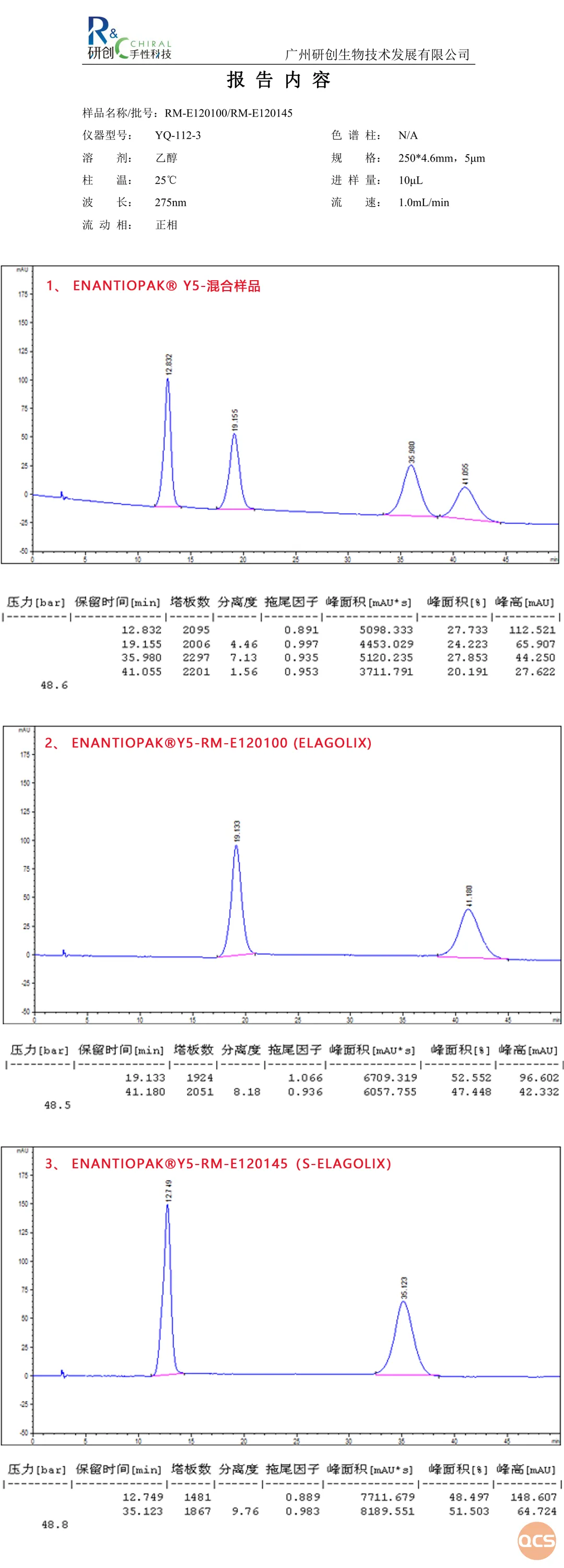
Figure 7: chiral separation data for Elagolix and S-Elagolix
The results of the chiral analysis reveal intriguing findings: both Elagolix and S-Elagolix exhibit double peaks, demonstrating effective separation. Recently, we received feedback from customers indicating the presence of two peaks in the chiral test between the product and its corresponding isomer. Consequently, we conducted an investigation into the unique characteristics of this product and ultimately identified that the answer lies within the blocking isomer. A detailed analysis will be presented in the upcoming issue of A Brief Introduction to Steric Photoactive Isomers Using Elagolix and Chiral Isomers as Examples.
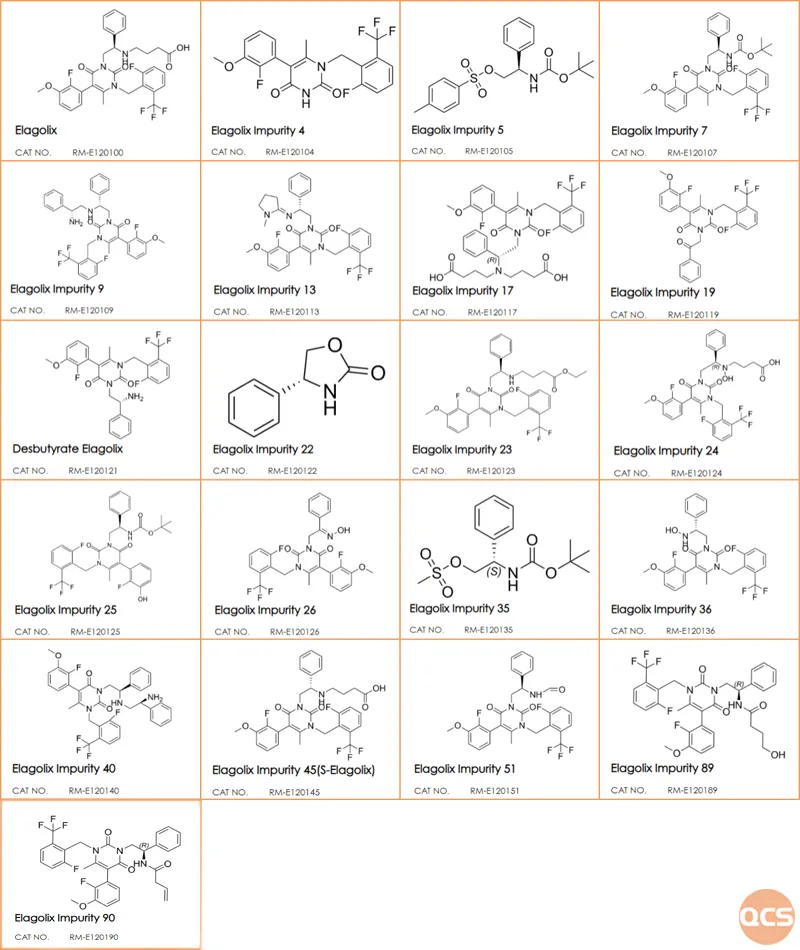
Figure 8: structural information on impurities in mixed sample data
The QCS R&D Center is equipped with advanced chiral testing equipment and a variety of chiral chromatography columns, effectively addressing the requirements of chiral research. Concurrently, the QCS Standard Material R&D Center collaborates closely with Guangzhou Research& Creative Biotechnology Co., Ltd. in the detection and separation of chiral compounds. Should you encounter analogous challenges, we invite you to engage in discussions with us, enabling a collaborative approach to addressing the complexities inherent in drug production and research. Our commitment to rigorous quality control is paramount to ensuring that effective pharmaceuticals are accessible for public use.
Press and hold the identification QR code to access a comprehensive list of all impurities.


Introduction: Today, we present an impurity study on a relatively niche pharmaceutical compound - Elagolix. This particular variant is being discussed primarily due to the client's observation of anomalous results in the detection of the corresponding isomer of Elagolix. We will elaborate on the irregularities encountered.
Elagolix (trade name: Orilissa) is an oral gonadotropin-releasing hormone (GnRH) antagonist that received approval from the US FDA in 2018 for the management of moderate to severe pain associated with endometriosis, specifically dysmenorrhea.
Currently, over 90 Elagolix impurities have been cataloged on the official QCS website (please scan the QR code at the end of this article to access the complete list of impurities). Our center has performed a liquid chromatographic characterization study on these impurities in accordance with our company's internal control standards for raw materials. The specific chromatographic methodology is illustrated in FIG. 1, while the results from mixed injection liquid chromatography are presented in FIG. 2.

Figure 1: regulation of chromatographic conditions internally

Figure 2: Mixed injection Figure 1-275nm
Figure 3 illustrates the detection of distinct wavelengths utilizing a uniform methodology for mixed samples:

Figure 3: Mixed injection Figure 2-210nm
As illustrated in Figures 2 and 3, nearly all impurities exhibit a significant degree of separation from API (RM-E120100). Figure 3 demonstrates that Elagolix (RM-E120100) and S-Elagolix (RM-E120145) overlap; subsequently, we combined the samples using the same methodology, with the corresponding isomers presented in Figure 4 and their structural formulas depicted in Figure 5.

Figure 4: schematic representation of the mixed injection process for Elagolix and its corresponding isomer, S-Elagolix

Figure 5: structural formula of Elagolix and its corresponding isomer,S-Elagolix
As illustrated in FIG. 4, Elagolix and S-Elagolix exhibit complete congruence within the inverted phase system. Subsequently, we re-evaluated the optical rotation data for both Elagolix and S-Elagolix, with the specific results presented in FIG. 6.

Figure 6: optical rotation data for Elagolix (RM-E120100) and S-Elagolix (RM-E120145)
Astute readers may question whether structural confirmation and optical rotation data alone are sufficient to ascertain the corresponding isomers. The conventional approach involves analyzing these isomers through chiral analytical methods. Consequently, our company has developed advanced chiral analysis techniques and successfully separated and tested the products through Chiral Standard Impurity Laboratory of QCS and R&Chiral.

Figure 7: chiral separation data for Elagolix and S-Elagolix
The results of the chiral analysis reveal intriguing findings: both Elagolix and S-Elagolix exhibit double peaks, demonstrating effective separation. Recently, we received feedback from customers indicating the presence of two peaks in the chiral test between the product and its corresponding isomer. Consequently, we conducted an investigation into the unique characteristics of this product and ultimately identified that the answer lies within the blocking isomer. A detailed analysis will be presented in the upcoming issue of A Brief Introduction to Steric Photoactive Isomers Using Elagolix and Chiral Isomers as Examples.

Figure 8: structural information on impurities in mixed sample data
The QCS R&D Center is equipped with advanced chiral testing equipment and a variety of chiral chromatography columns, effectively addressing the requirements of chiral research. Concurrently, the QCS Standard Material R&D Center collaborates closely with Guangzhou Research& Creative Biotechnology Co., Ltd. in the detection and separation of chiral compounds. Should you encounter analogous challenges, we invite you to engage in discussions with us, enabling a collaborative approach to addressing the complexities inherent in drug production and research. Our commitment to rigorous quality control is paramount to ensuring that effective pharmaceuticals are accessible for public use.
Press and hold the identification QR code to access a comprehensive list of all impurities.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号