Time:2023-06-13
Introduction: Today, we will share the difficulties encountered in the synthesis, purification, and analysis of Fungal drug-Amorolfine impurity.
Amorlfine Hydrochloride (trade name: Loceryl) is commonly used in cream and ointment formulations. Used for skin fungal diseases caused by skin fungi, such as tinea pedis (athlete's foot), tinea pedis, tinea corporis, and candidiasis. At present, according to data from yazhit.com, there have been no generic drugs that have been evaluated. From internal data, there are no less than 10 domestic companies working on this product. We will wait and see who will be the first to be evaluated.
The QCS website currently has about 20 impurities in Amorlfine (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the European Pharmacopoeia (EP) standard for Amorlfine Hydrochloride and focused on locating several specific impurities (Imp D, Imp E, Imp I, Imp J, Imp M) and some non-specific impurities included in the EP. Firstly, a summary of the mixed injection data of several specific impurities (Imp D, Imp E, Imp I, Imp J, Imp M) and API is shown in Figure 1:
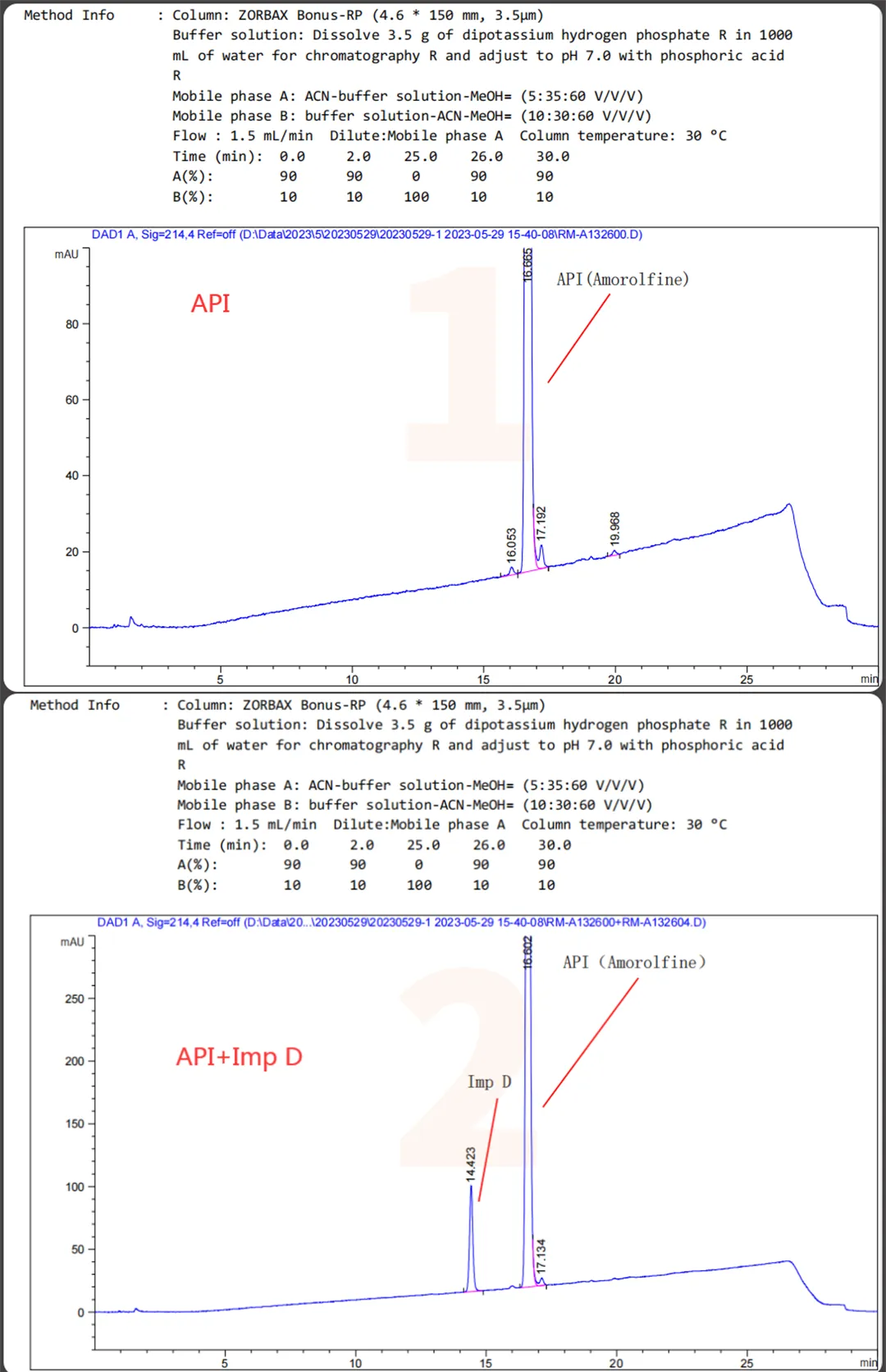
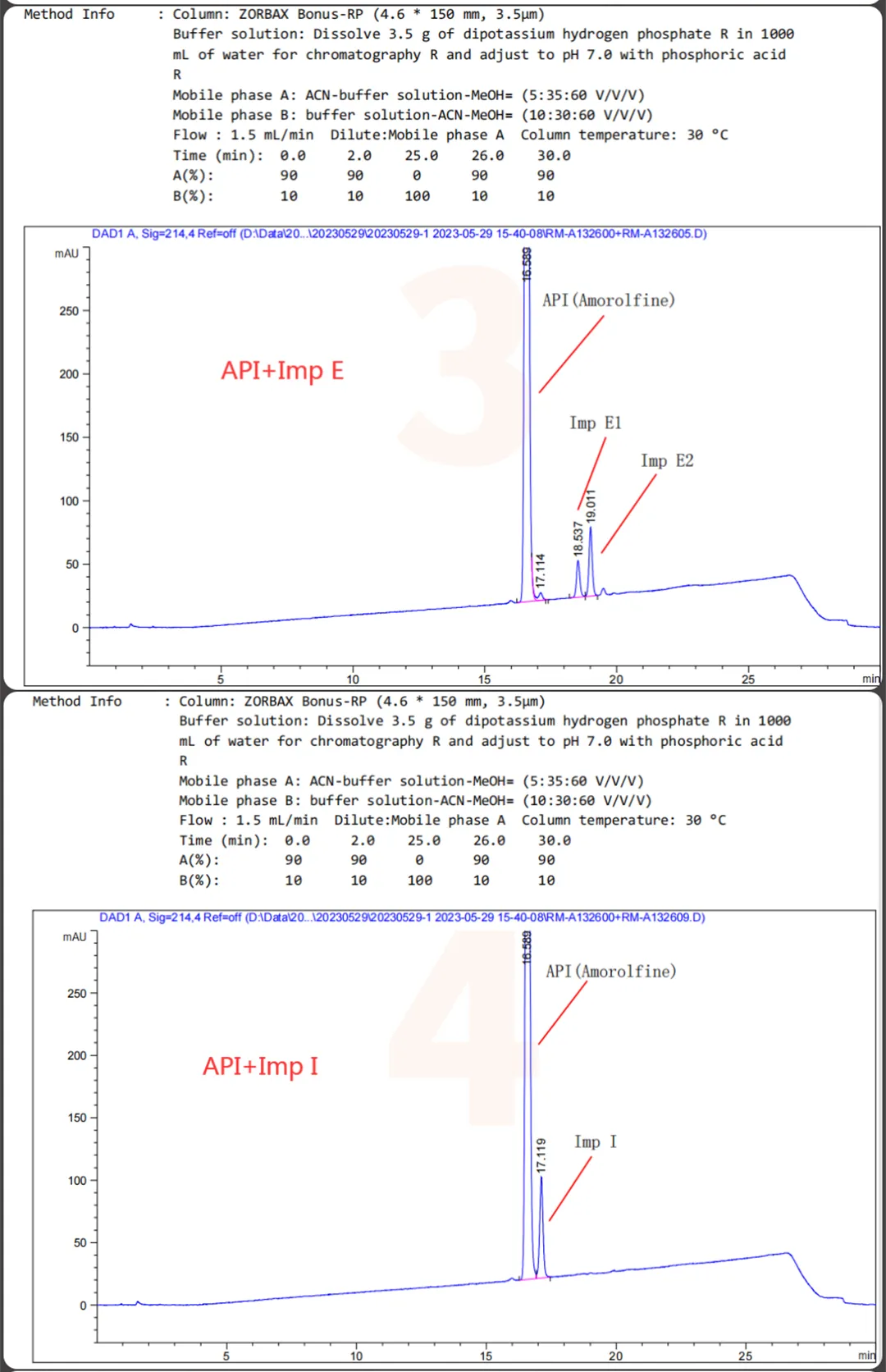
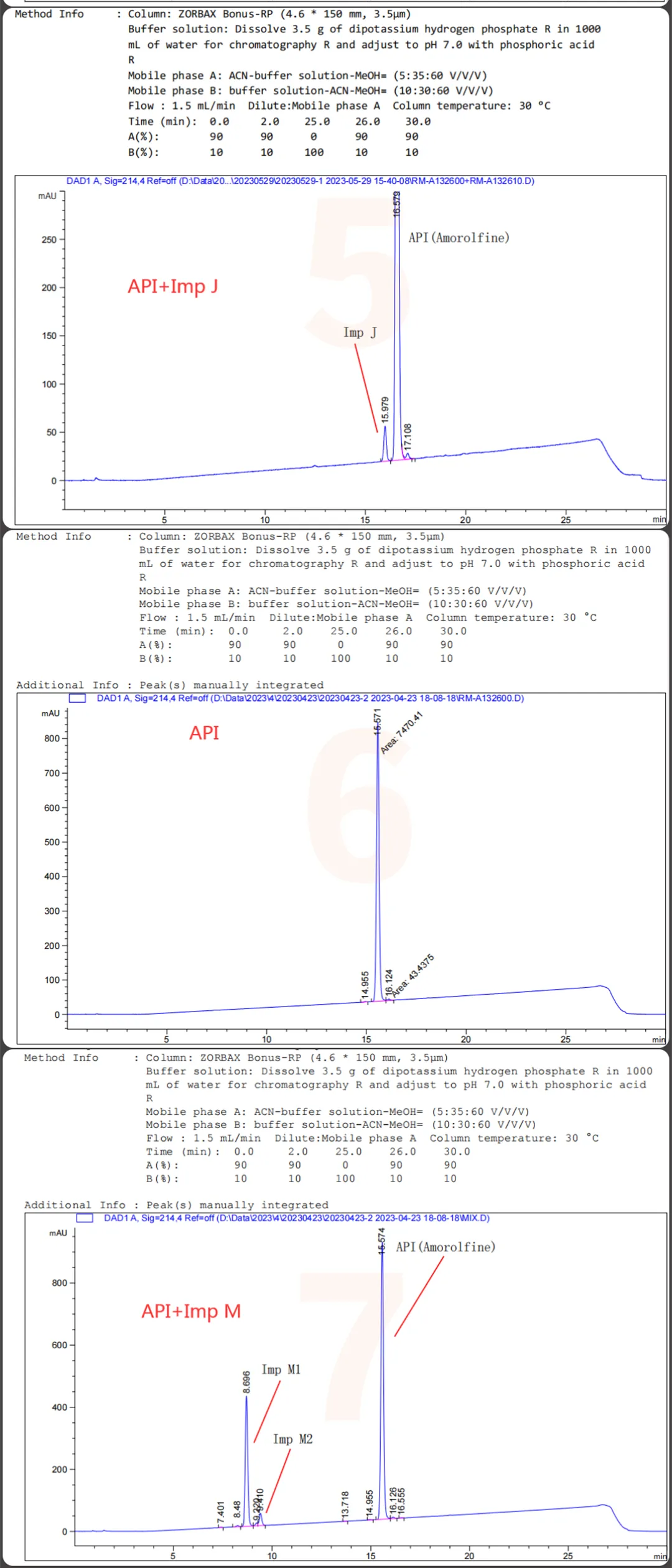
Figure 1: Mixing of specific impurities and API into Sample
Through the above four specific impurity localization studies, it can be found that they are completely consistent with the EP specification data. The RRT specification for the four impurities in the EP standard is shown in Figure 2:
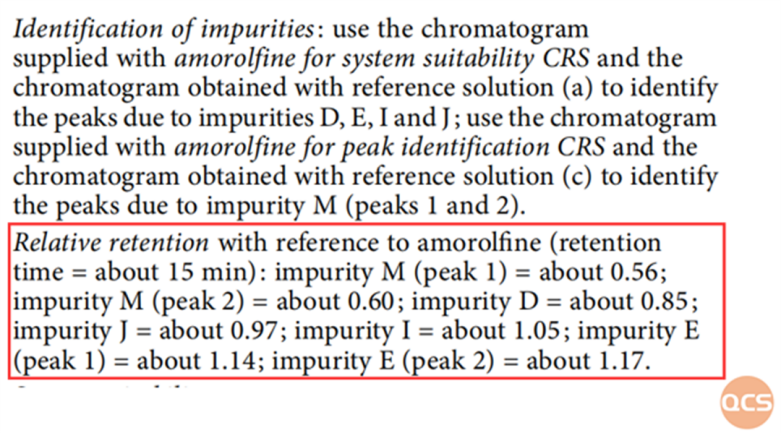
Figure 2: Theoretical data of relative retention time between specific impurities and API Data source: EP 10.0
Observant friends may have noticed that impurity DEI and M were not tested at the same time. Impurity M was already validated during shipment, so the need for further sampling validation was eliminated during the same testing. Now summarize the mixed injection data of impurities and APIs in Figure 1, as shown in Figure 3:
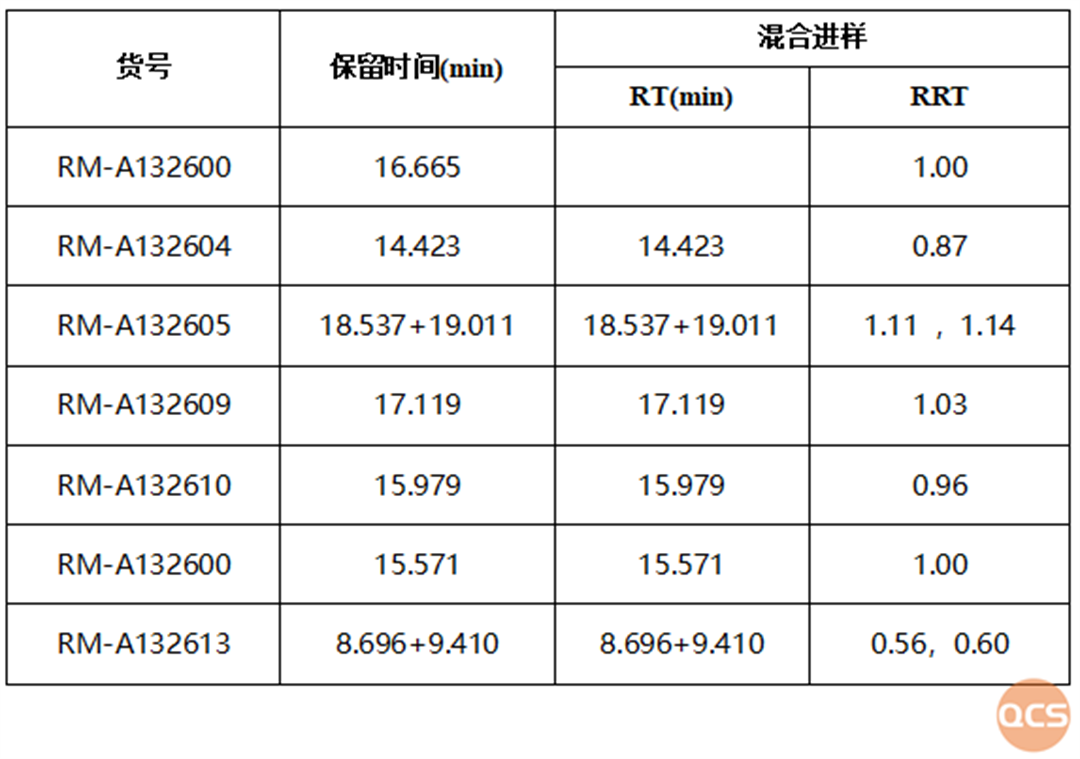
Figure 3: Summary of mixed injection data of specific impurities and APIs
It can be seen from the summary table that when impurity M was shipped in April, in order to verify the accuracy of the product, the same method as the EP standard was used, and the RRT data located was the same as that specified in the pharmacopoeia. On May 29th, when conducting method validation on other impurities together, it was found that the same method had a slight difference between DEIJ's RRT and EP standard inclusion. Comparing the API data before and after, it can be seen that the column efficiency decreased and the detection effect of the product was also poor.
This issue has caused some difficulties in the production of impurity B (RM-A132602) in Amorlfine EP. Amorlfine impurity B is a product containing formyl and acetyl structures. After careful design by the synthesis researchers at QCS R&D center, a synthesis design with a total of seven steps but only requiring three separations and purifications (see Figure 4) was quickly achieved, resulting in highly selective and high-yield directional synthesis of impurity B in Amorofen EP. However, during the qualitative and quantitative testing of the synthesized product, the performance of the chromatographic column decreased, resulting in a false impression of retention time drift and liquid phase purity distortion, which delayed the acceptance of the product. Finally, through the real-time detection of the QCS R&D center's own nuclear magnetic equipment, abnormalities were found in the test samples during the use of the chromatographic column. After timely replacement of the chromatographic column, correct data was obtained, ensuring the effective supply of the Amorlfine related substance series products.
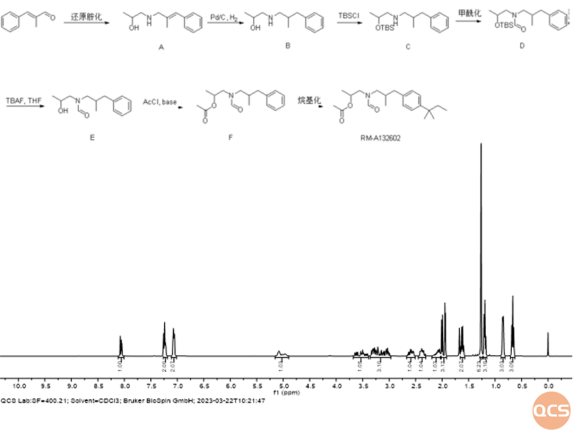
Figure 4: Synthesis process and nuclear magnetic resonance data of impurity B in Amorlfine
Finally, the localization maps and data of non-specific impurities included in other EPs will be summarized and shared as shown in Figure 5:
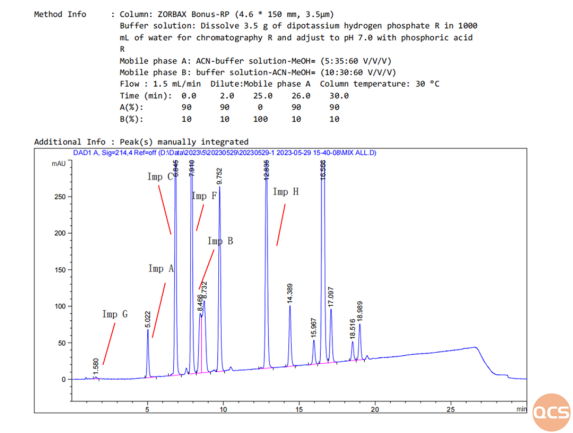
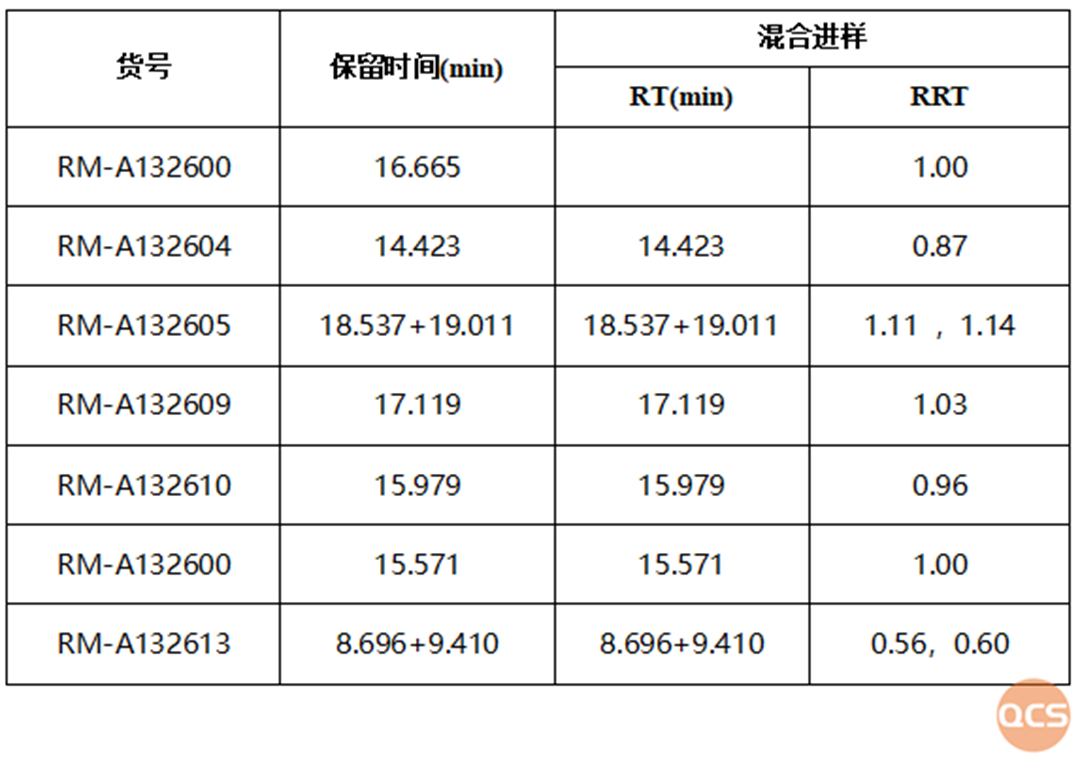
Figure 5: Product mix graph and data summary table
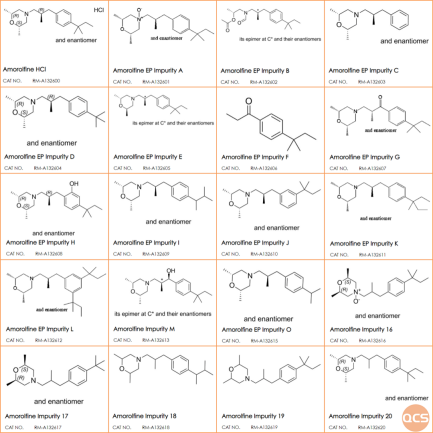
Figure 6: Structural information of related products
QCS has completed the development of all standard impurities for Amorlfine EP after half a year. Please feel free to inquire or request samples from our company.
Long press to recognize the QR code and view the list of all impurities!


Introduction: Today, we will share the difficulties encountered in the synthesis, purification, and analysis of Fungal drug-Amorolfine impurity.
Amorlfine Hydrochloride (trade name: Loceryl) is commonly used in cream and ointment formulations. Used for skin fungal diseases caused by skin fungi, such as tinea pedis (athlete's foot), tinea pedis, tinea corporis, and candidiasis. At present, according to data from yazhit.com, there have been no generic drugs that have been evaluated. From internal data, there are no less than 10 domestic companies working on this product. We will wait and see who will be the first to be evaluated.
The QCS website currently has about 20 impurities in Amorlfine (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the European Pharmacopoeia (EP) standard for Amorlfine Hydrochloride and focused on locating several specific impurities (Imp D, Imp E, Imp I, Imp J, Imp M) and some non-specific impurities included in the EP. Firstly, a summary of the mixed injection data of several specific impurities (Imp D, Imp E, Imp I, Imp J, Imp M) and API is shown in Figure 1:



Figure 1: Mixing of specific impurities and API into Sample
Through the above four specific impurity localization studies, it can be found that they are completely consistent with the EP specification data. The RRT specification for the four impurities in the EP standard is shown in Figure 2:

Figure 2: Theoretical data of relative retention time between specific impurities and API Data source: EP 10.0
Observant friends may have noticed that impurity DEI and M were not tested at the same time. Impurity M was already validated during shipment, so the need for further sampling validation was eliminated during the same testing. Now summarize the mixed injection data of impurities and APIs in Figure 1, as shown in Figure 3:

Figure 3: Summary of mixed injection data of specific impurities and APIs
It can be seen from the summary table that when impurity M was shipped in April, in order to verify the accuracy of the product, the same method as the EP standard was used, and the RRT data located was the same as that specified in the pharmacopoeia. On May 29th, when conducting method validation on other impurities together, it was found that the same method had a slight difference between DEIJ's RRT and EP standard inclusion. Comparing the API data before and after, it can be seen that the column efficiency decreased and the detection effect of the product was also poor.
This issue has caused some difficulties in the production of impurity B (RM-A132602) in Amorlfine EP. Amorlfine impurity B is a product containing formyl and acetyl structures. After careful design by the synthesis researchers at QCS R&D center, a synthesis design with a total of seven steps but only requiring three separations and purifications (see Figure 4) was quickly achieved, resulting in highly selective and high-yield directional synthesis of impurity B in Amorofen EP. However, during the qualitative and quantitative testing of the synthesized product, the performance of the chromatographic column decreased, resulting in a false impression of retention time drift and liquid phase purity distortion, which delayed the acceptance of the product. Finally, through the real-time detection of the QCS R&D center's own nuclear magnetic equipment, abnormalities were found in the test samples during the use of the chromatographic column. After timely replacement of the chromatographic column, correct data was obtained, ensuring the effective supply of the Amorlfine related substance series products.

Figure 4: Synthesis process and nuclear magnetic resonance data of impurity B in Amorlfine
Finally, the localization maps and data of non-specific impurities included in other EPs will be summarized and shared as shown in Figure 5:


Figure 5: Product mix graph and data summary table

Figure 6: Structural information of related products
QCS has completed the development of all standard impurities for Amorlfine EP after half a year. Please feel free to inquire or request samples from our company.
Long press to recognize the QR code and view the list of all impurities!


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号