Time:2023-05-23
Introduction
Currently, there are nearly 50 domestic enterprises engaged in the research of this variety of preparations and raw materials, with approximately 30 of these companies having undergone evaluation (including original research). Furthermore, 11 raw materials have been transferred to A. The QCS R&D Center has observed that several customers' Dapoxetine tablet projects have recently commenced their transition into production. Today, we aim to share with our clients the primary impurities studied during the consistency evaluation process and provide qualitative liquid chromatography data regarding this series of impurities. Let us examine whether any impurities may have been overlooked.
INN Dapoxetine hydrochloride tablets (drug name:Priligy, ) are rapid-acting selective serotonin reuptake inhibitors (SSRIs) indicated for the treatment of premature ejaculation and erectile dysfunction in males.
Currently, a total of 78 Dapoxetine impurities are listed on the official QCS website (please scan the QR code at the end of this article to access the complete list of impurities). Our center adheres to the preparation standard for Dapoxetine hydrochloride tablets (standard number: JX20150184), which includes an analysis of over 20 frequently procured spot impurities using liquid chromatography (refer to Figure 6 for a summary table and structural formulas). The specific results from mixed injection liquid chromatography are presented in Figures 1 and 2.
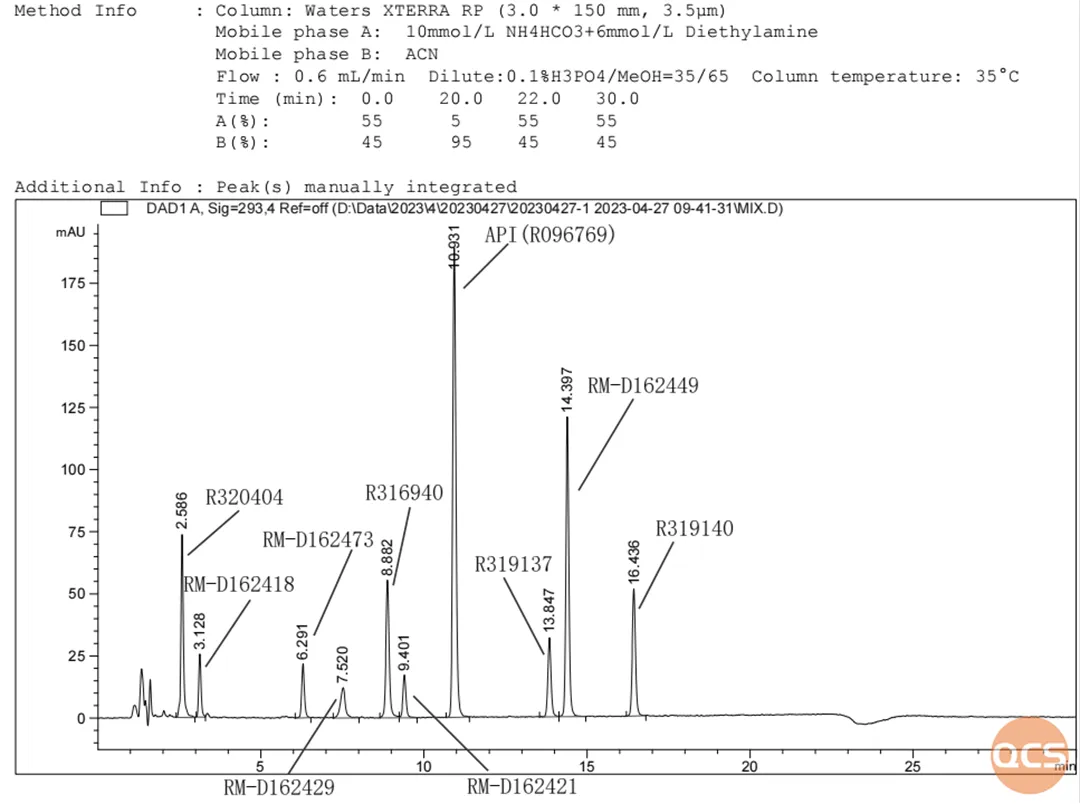
Figure 1: Mixing sample at a wavelength of 293 nm
Data source: QCS Standard Materials Research and Development Center
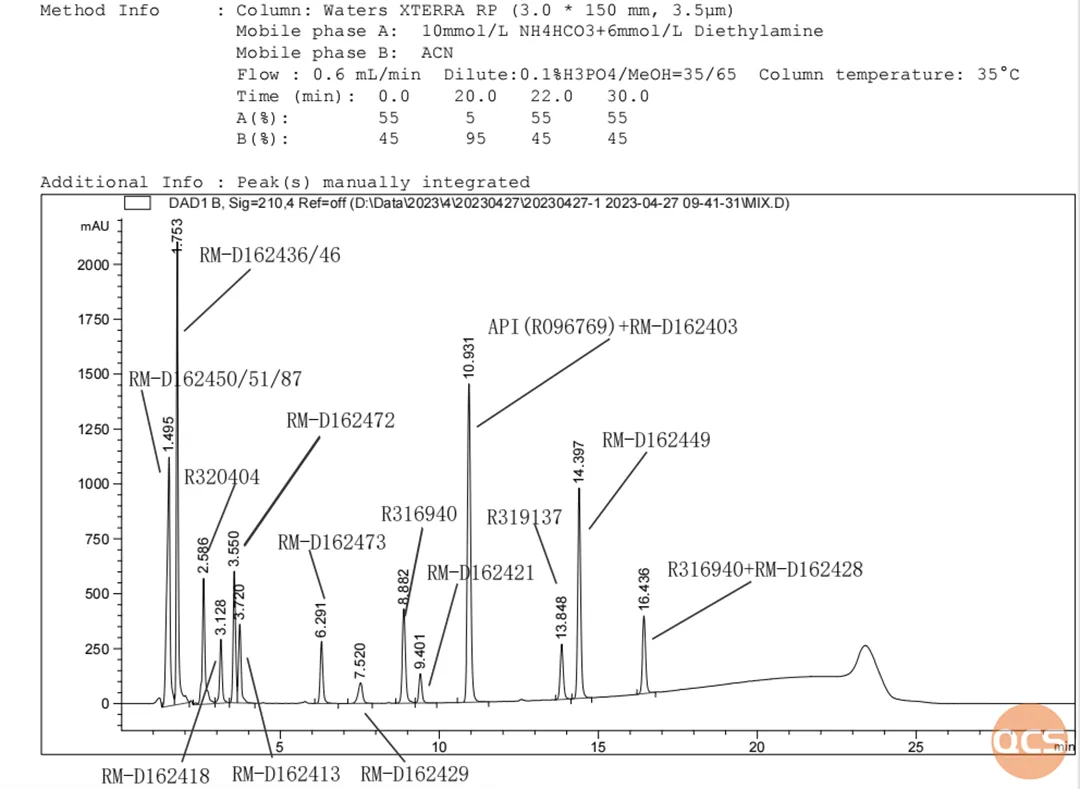
Figure 2: Mixed sampling of 18 products at a wavelength of 210 nm
Data source: QCS Standard Materials Research and Development Center
The similar structural characteristics of several impurity products pose a challenge for their separation under the current chromatographic conditions. For instance, as illustrated in FIG. 2, RM-D162450, RM-D162451, and RM-D162487 exhibit overlapping peaks; similarly, RM-D162436 and RM-D162446 overlap with each other, while RM-D162406 and RM-D162428 also show overlap. Additionally, API (R096769) overlaps with RM-D162403, resulting in only four distinct peaks among the nine products.
The chemists from the Analysis Department of the QCS R&D Center further identified nine products (four peaks) exhibiting overlapping chromatographic peaks by comparing data from individual samples with that from mixed samples. As illustrated in Figure 3, although the chemical properties of these products are closely related, there are subtle differences in the retention times of various compounds within the individual sample data. Notably, the two sets of signals corresponding to RM-D162436 and RM-D162446 remain indistinguishable. The structural formulas indicate that compounds 36 and 46 are enantiomers, which cannot be differentiated using conventional reverse-phase systems; however, chiral chromatography may be employed for identification if necessary. The results and data pertaining to these product samples are presented in Figures 3 and 4.
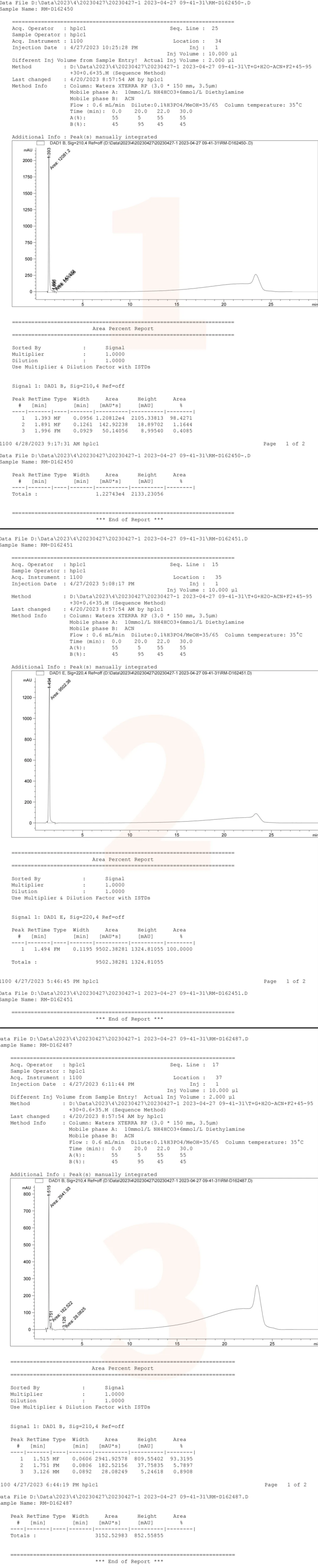
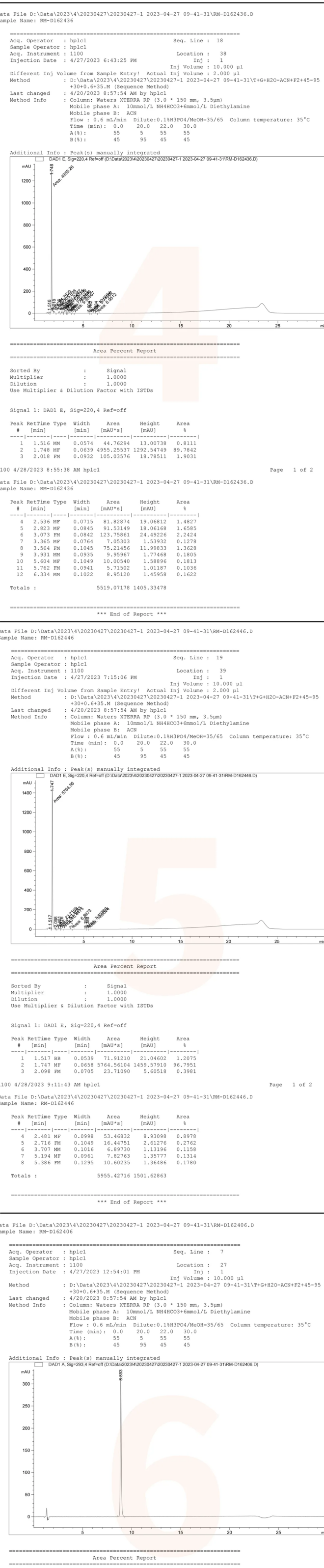
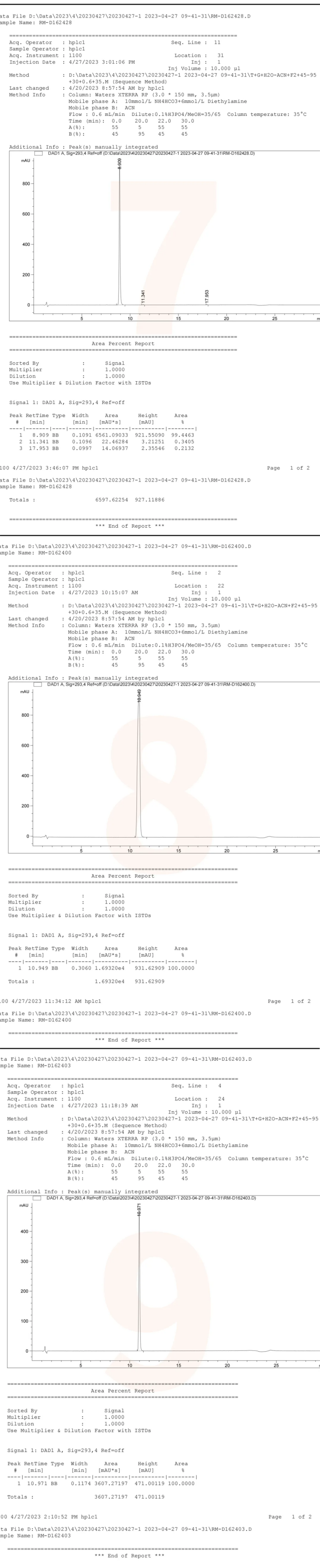
Figure 3: Nine distinct product samples are illustrated in Figure 3
Data source: QCS Standard Materials Research and Development Center
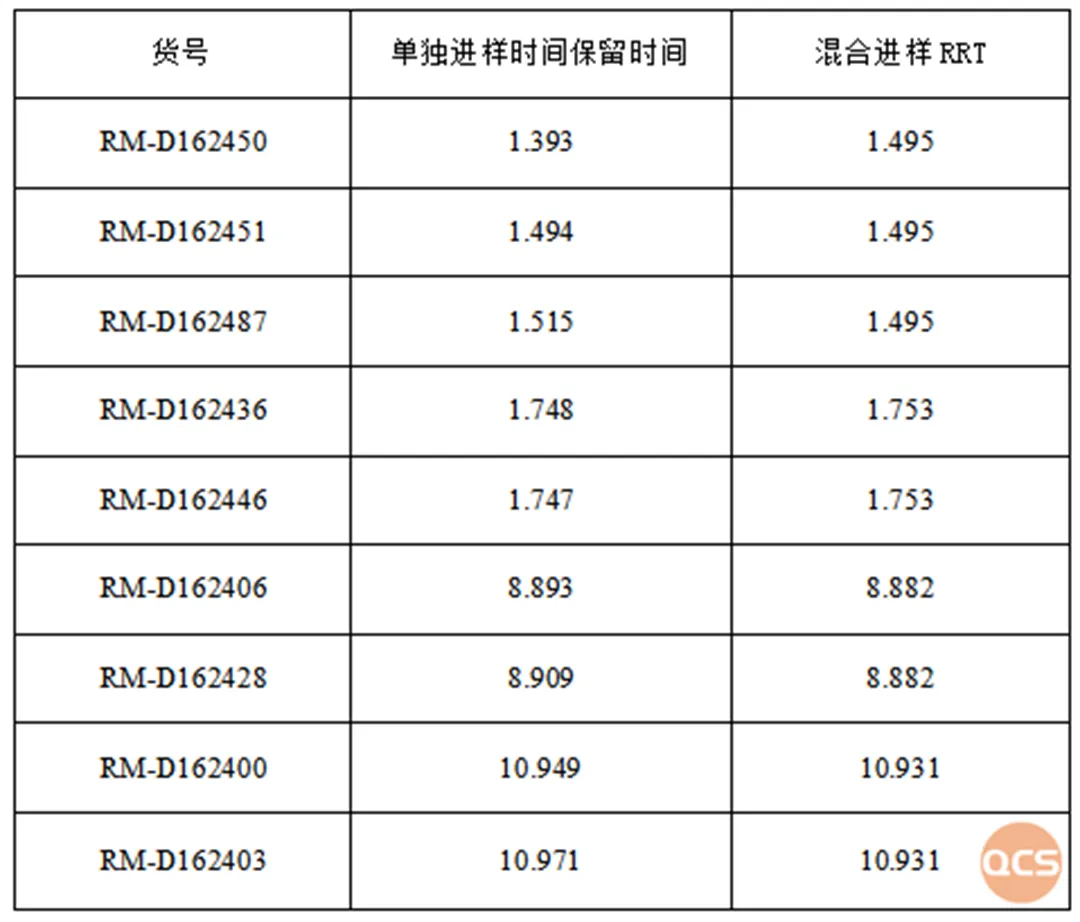
Figure 4: Overview of data from nine distinct samples
Data source: QCS Standard Materials Research and Development Center
Considering the aforementioned factors, several products can be differentiated under individual injection conditions; however, achieving effective separation through mixed injection poses significant challenges. Therefore, it may be necessary to specifically target these Dapoxetine hydrochloride impurities in the development of analytical methods throughout the research process.
The Dapoxetine hydrochloride series impurities, developed earlier by the QCS R&D center, have allowed us to accumulate extensive research data and product samples during our product development phase. The impurities produced by our team have supported the impurity research efforts of numerous reviewed enterprises. As this drug variety has entered national procurement lists, coupled with an increase in patient demand and an expansion of production capacity, we anticipate a growing need for specific impurity research within the quality control processes of pharmaceutical companies. The QCS R&D center will continue to provide Dapoxetine hydrochloride impurity products and services to assist each customer in enhancing drug quality.
Product information
The summary of the product mixing sampling data is presented in Figure 5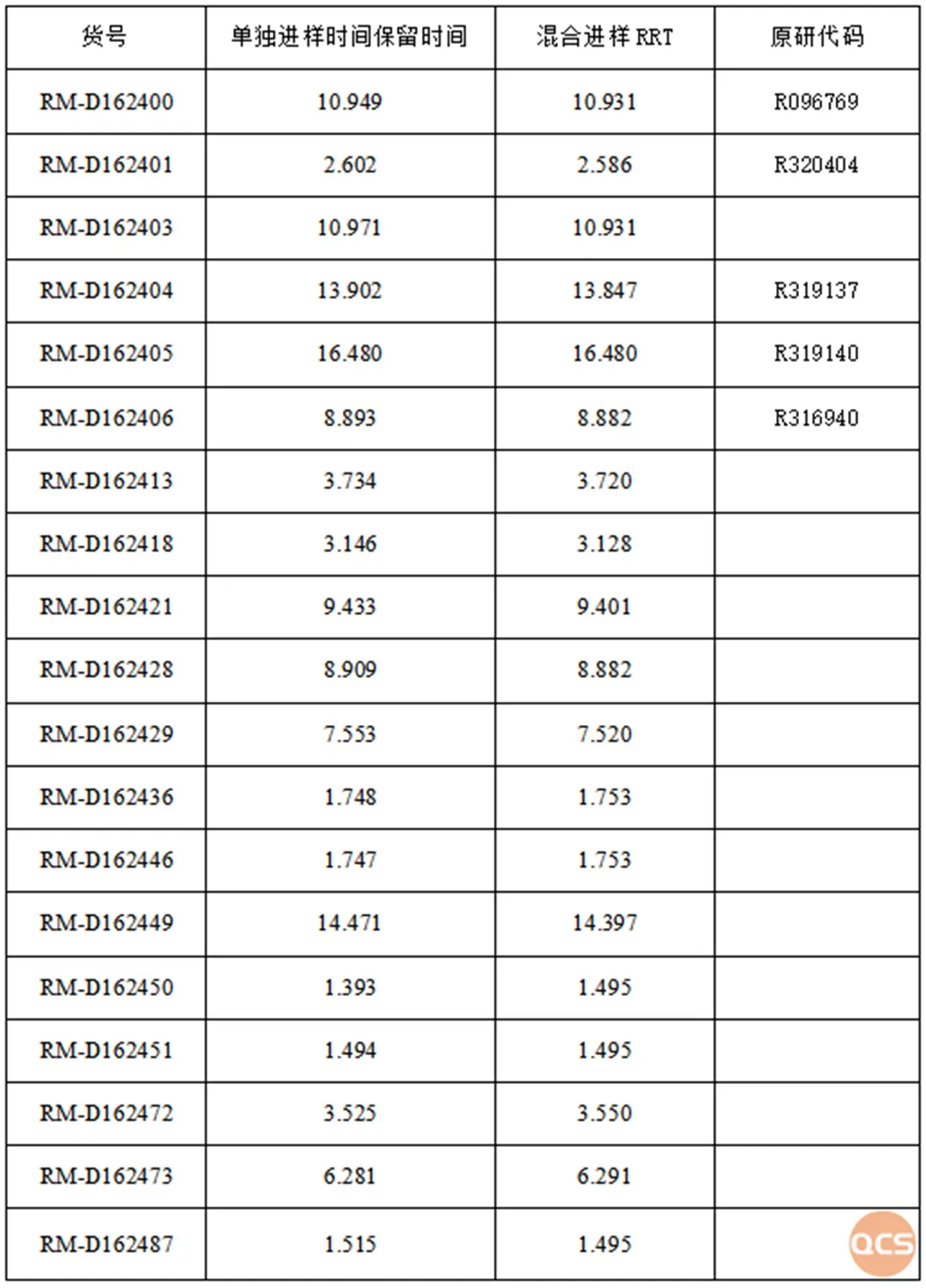
Figure 5: Overview of product mix sampling data
Data source: QCS Standard Materials Research and Development Center
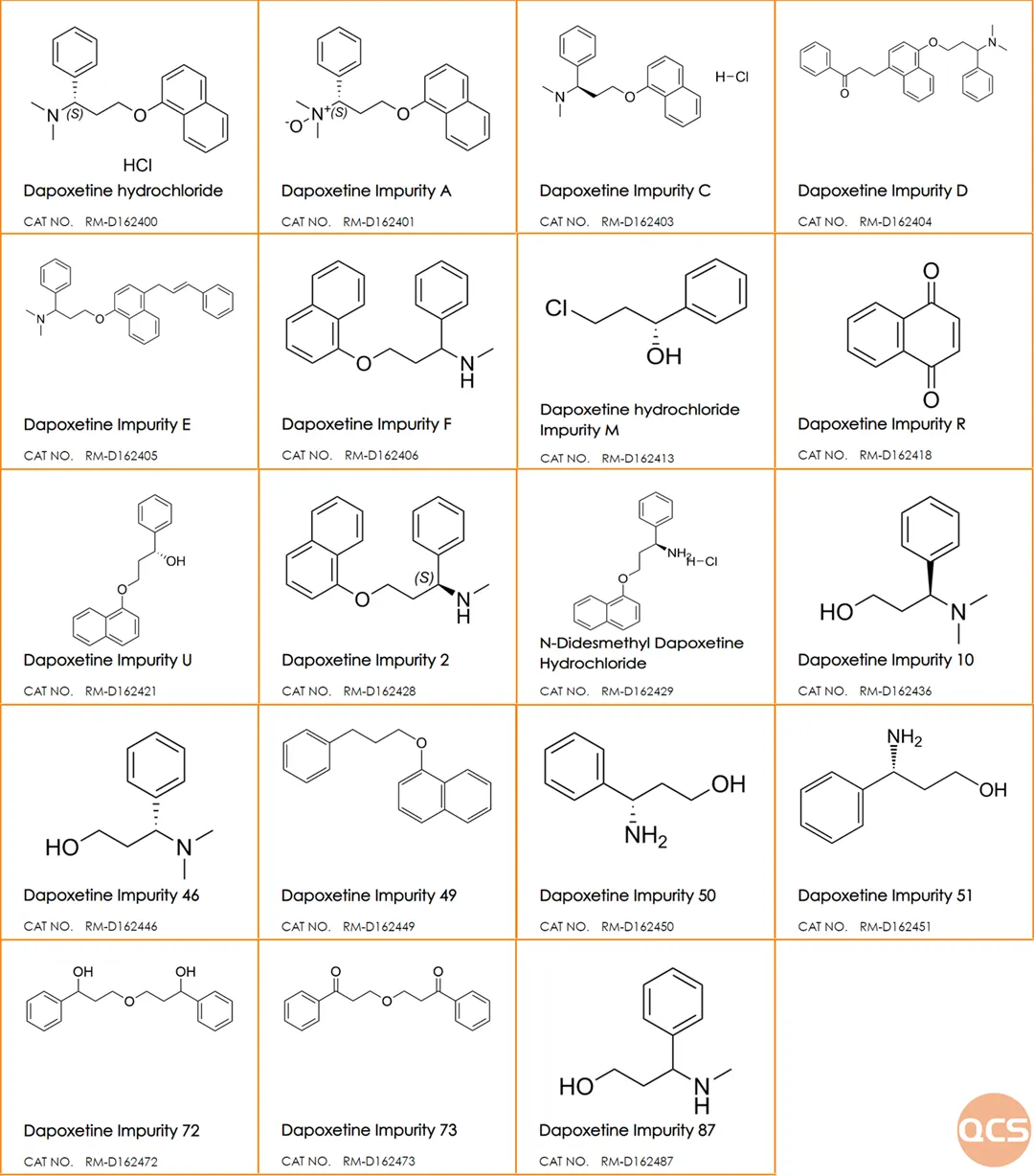
Figure 6: Information on the relevant product structure
Data source: QCS Standard Materials Research and Development Center
Press and hold the identification QR code to access a comprehensive list of all impurities.


Introduction
Currently, there are nearly 50 domestic enterprises engaged in the research of this variety of preparations and raw materials, with approximately 30 of these companies having undergone evaluation (including original research). Furthermore, 11 raw materials have been transferred to A. The QCS R&D Center has observed that several customers' Dapoxetine tablet projects have recently commenced their transition into production. Today, we aim to share with our clients the primary impurities studied during the consistency evaluation process and provide qualitative liquid chromatography data regarding this series of impurities. Let us examine whether any impurities may have been overlooked.
INN Dapoxetine hydrochloride tablets (drug name:Priligy, ) are rapid-acting selective serotonin reuptake inhibitors (SSRIs) indicated for the treatment of premature ejaculation and erectile dysfunction in males.
Currently, a total of 78 Dapoxetine impurities are listed on the official QCS website (please scan the QR code at the end of this article to access the complete list of impurities). Our center adheres to the preparation standard for Dapoxetine hydrochloride tablets (standard number: JX20150184), which includes an analysis of over 20 frequently procured spot impurities using liquid chromatography (refer to Figure 6 for a summary table and structural formulas). The specific results from mixed injection liquid chromatography are presented in Figures 1 and 2.

Figure 1: Mixing sample at a wavelength of 293 nm
Data source: QCS Standard Materials Research and Development Center

Figure 2: Mixed sampling of 18 products at a wavelength of 210 nm
Data source: QCS Standard Materials Research and Development Center
The similar structural characteristics of several impurity products pose a challenge for their separation under the current chromatographic conditions. For instance, as illustrated in FIG. 2, RM-D162450, RM-D162451, and RM-D162487 exhibit overlapping peaks; similarly, RM-D162436 and RM-D162446 overlap with each other, while RM-D162406 and RM-D162428 also show overlap. Additionally, API (R096769) overlaps with RM-D162403, resulting in only four distinct peaks among the nine products.
The chemists from the Analysis Department of the QCS R&D Center further identified nine products (four peaks) exhibiting overlapping chromatographic peaks by comparing data from individual samples with that from mixed samples. As illustrated in Figure 3, although the chemical properties of these products are closely related, there are subtle differences in the retention times of various compounds within the individual sample data. Notably, the two sets of signals corresponding to RM-D162436 and RM-D162446 remain indistinguishable. The structural formulas indicate that compounds 36 and 46 are enantiomers, which cannot be differentiated using conventional reverse-phase systems; however, chiral chromatography may be employed for identification if necessary. The results and data pertaining to these product samples are presented in Figures 3 and 4.



Figure 3: Nine distinct product samples are illustrated in Figure 3
Data source: QCS Standard Materials Research and Development Center

Figure 4: Overview of data from nine distinct samples
Data source: QCS Standard Materials Research and Development Center
Considering the aforementioned factors, several products can be differentiated under individual injection conditions; however, achieving effective separation through mixed injection poses significant challenges. Therefore, it may be necessary to specifically target these Dapoxetine hydrochloride impurities in the development of analytical methods throughout the research process.
The Dapoxetine hydrochloride series impurities, developed earlier by the QCS R&D center, have allowed us to accumulate extensive research data and product samples during our product development phase. The impurities produced by our team have supported the impurity research efforts of numerous reviewed enterprises. As this drug variety has entered national procurement lists, coupled with an increase in patient demand and an expansion of production capacity, we anticipate a growing need for specific impurity research within the quality control processes of pharmaceutical companies. The QCS R&D center will continue to provide Dapoxetine hydrochloride impurity products and services to assist each customer in enhancing drug quality.
Product information
The summary of the product mixing sampling data is presented in Figure 5
Figure 5: Overview of product mix sampling data
Data source: QCS Standard Materials Research and Development Center

Figure 6: Information on the relevant product structure
Data source: QCS Standard Materials Research and Development Center
Press and hold the identification QR code to access a comprehensive list of all impurities.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号