Time:2023-04-21
Introduction: Simcere Pharmaceutical recently announced its 2022 performance, with data showing that its total revenue in 2022 was 6.319 billion yuan, with innovative drug revenue accounting for 65.3%. It was also mentioned in the article that among the innovative drug business segments, Simcere's revenue in the field of autoimmunity was about 1.28 billion yuan, with a year-on-year growth of 39.4%. Its main products were Iremod (Iguratimod Tablets), Antine (Diclofenac Sodium Sustained Release Capsules/gel), etc. In this issue, we will share our research ideas and qualitative research data on impurities in Iguratimod.
Iguratimod tablets (product name: Iremod  ) In China, it was first developed by Tianjin Pharmaceutical Research Institute and Simcere Pharmaceutical in 2003. In 2004, it obtained clinical approval from CDE. In January 2008, Simcere
) In China, it was first developed by Tianjin Pharmaceutical Research Institute and Simcere Pharmaceutical in 2003. In 2004, it obtained clinical approval from CDE. In January 2008, Simcere
Pharmaceutical completed clinical research and submitted an NDA to the National Medical Products Administration. In August 2011, it was approved for market launch under the brand name Iremod  . Mainly used for treating active
. Mainly used for treating active
rheumatoid arthritis.
There are a total of 47 impurities related to the raw materials of Iguratimod (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the relevant standards of Iguratimod raw materials and conducted liquid chromatography research on more than 20 selected spot products (data summary table and structural formula are shown in Figure 6). The specific mixed injection liquid chromatography results are shown in Figure 1
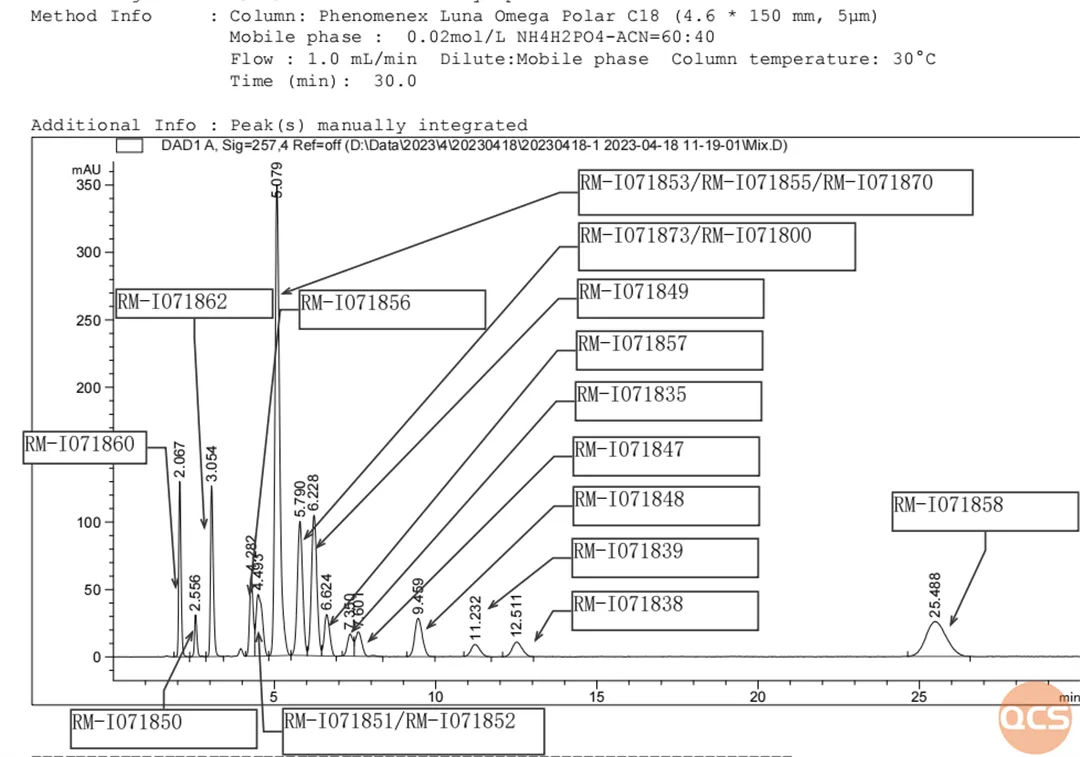
Figure 1: Mixed sample figure 1
Data source: QCS Standard Material R&D Center
Due to the similar structure of multiple impurity products, there may be difficulties in separating them under this chromatographic condition. For example, in Figure 1, we can see that RM-I071851 and RM-I071852 overlap, RM-I071873 and RM-I071800 overlap, and RM-I071870 and RM-I0718755 overlap with RM-I071853. Only three sets of peaks were observed for the seven impurities.
The experimental results show that reducing the injection volume can achieve certain differentiation between the two structural signals RM-I071851 and RM-I071852, but the separation effect is not significant; And the RM-I071873/RM-I071800 signals are still indistinguishable, reminding researchers to pay attention to the use of analytical methods in the study of the above impurities. The liquid chromatography results and data of the mixed injection are shown in Figures 2 and 3:
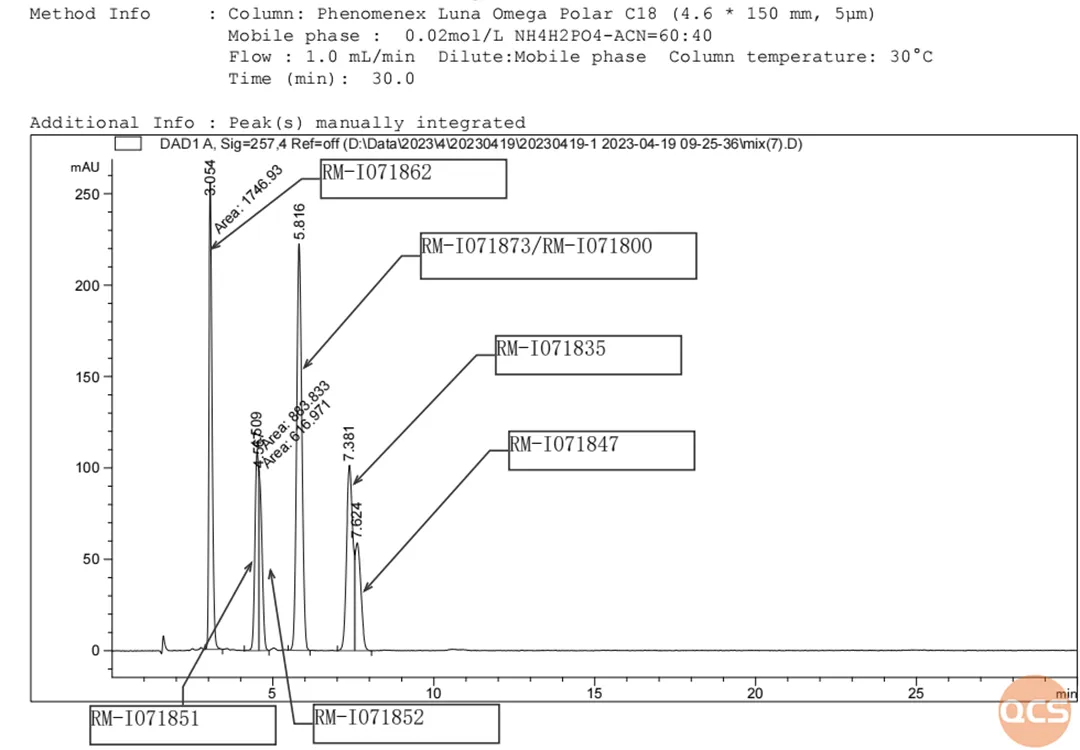
Figure 2: Mixed Sample Figure 2
Data source: QCS Standard Material R&D Center

Figure 3: Summary of mixed injection data in Figure 2
Data source: QCS Standard Material R&D Center
In addition, it should be noted that the four impurities RM-I071870/RM-I0718755/RM-I071853/RM-I071873 cannot be effectively distinguished from other impurities under this standard chromatographic method. From Figure 1 and Figure 2, it can be seen that the three impurities RM-I071870/RM-I0718755/RM-I071853 cannot be distinguished from each other under standard chromatographic conditions, and RM-I071873 and the active pharmaceutical ingredient RM-I071800 cannot be distinguished. Using this chromatographic condition to study the above four impurities may require further optimization of the analytical method. The following is a summary of the chromatographic results of individual injections of RM-I071870, RM-I0718755, RM-I071853, RM-I071873, and the active pharmaceutical ingredient RM-I071800.
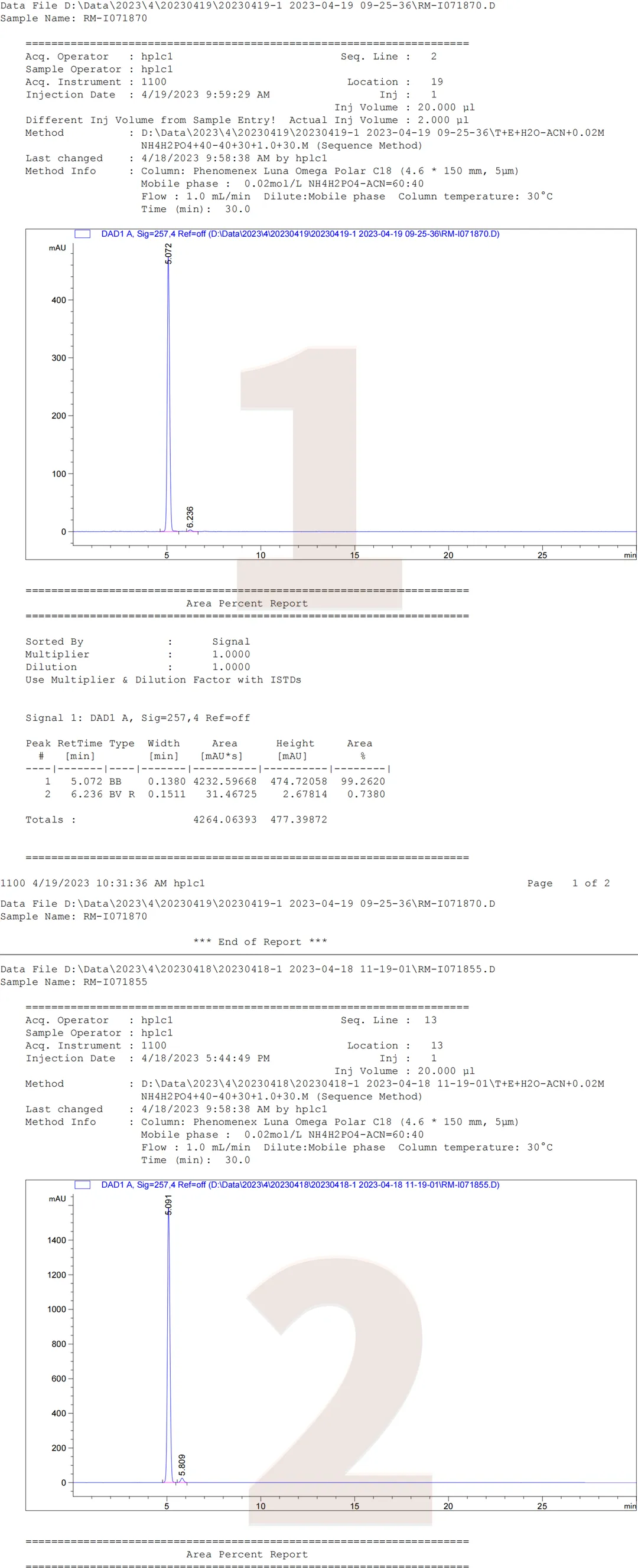

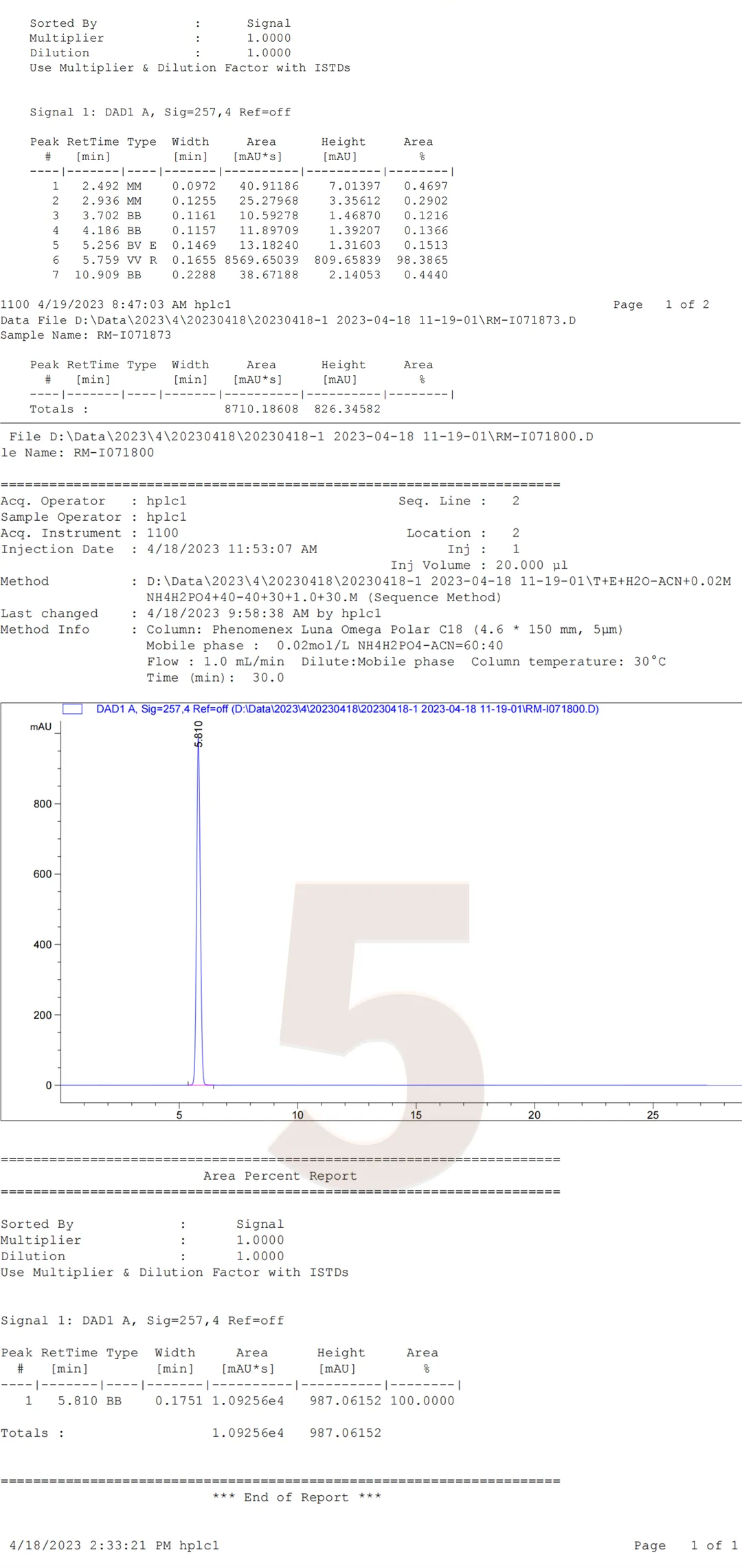
Figure 4: Five products individually sampled
Data source: QCS Standard Material R&D Center
The Iguratimod series impurities are a key variety developed by our company. After the full chain coordination of synthesis, analysis, and preparation by the company, the project took less than a month from the initial approval to the completion of the project. Since there is no relevant impurity information in the standard or literature, our company focuses on deducing the process and degradation impurities that may be generated in the process through in-depth study of the Iguratimod synthesis process. If you are interested in understanding how our company deduces and determines the above impurities, you can leave a comment on the official account. Later, we will make a special phase I of the ideas on the generation of all impurities and the pitfalls in the synthesis to give you a guide to avoid pitfalls.
Product Information
The summary table of product mixed injection data is shown in Figure 5:
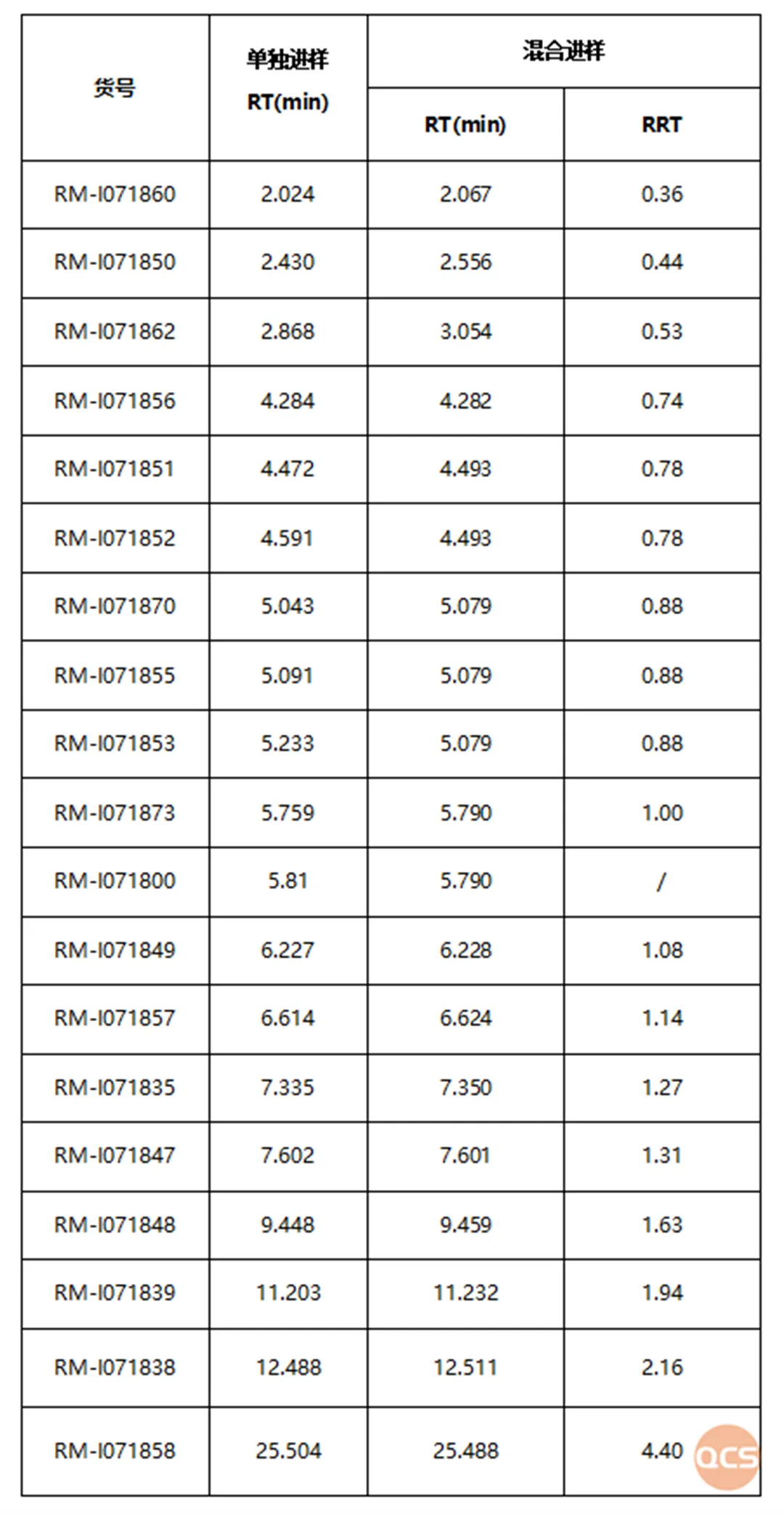
Figure 5: Summary table of product mixed injection data
Data source: QCS Standard Material R&D Center

Figure 6: Structural information of related products
Data source: QCS Standard Material R&D Center
Long press to recognize the QR code and view the list of all impurities!

Introduction: Simcere Pharmaceutical recently announced its 2022 performance, with data showing that its total revenue in 2022 was 6.319 billion yuan, with innovative drug revenue accounting for 65.3%. It was also mentioned in the article that among the innovative drug business segments, Simcere's revenue in the field of autoimmunity was about 1.28 billion yuan, with a year-on-year growth of 39.4%. Its main products were Iremod (Iguratimod Tablets), Antine (Diclofenac Sodium Sustained Release Capsules/gel), etc. In this issue, we will share our research ideas and qualitative research data on impurities in Iguratimod.
Iguratimod tablets (product name: Iremod  ) In China, it was first developed by Tianjin Pharmaceutical Research Institute and Simcere Pharmaceutical in 2003. In 2004, it obtained clinical approval from CDE. In January 2008, Simcere
) In China, it was first developed by Tianjin Pharmaceutical Research Institute and Simcere Pharmaceutical in 2003. In 2004, it obtained clinical approval from CDE. In January 2008, Simcere
Pharmaceutical completed clinical research and submitted an NDA to the National Medical Products Administration. In August 2011, it was approved for market launch under the brand name Iremod  . Mainly used for treating active
. Mainly used for treating active
rheumatoid arthritis.
There are a total of 47 impurities related to the raw materials of Iguratimod (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the relevant standards of Iguratimod raw materials and conducted liquid chromatography research on more than 20 selected spot products (data summary table and structural formula are shown in Figure 6). The specific mixed injection liquid chromatography results are shown in Figure 1

Figure 1: Mixed sample figure 1
Data source: QCS Standard Material R&D Center
Due to the similar structure of multiple impurity products, there may be difficulties in separating them under this chromatographic condition. For example, in Figure 1, we can see that RM-I071851 and RM-I071852 overlap, RM-I071873 and RM-I071800 overlap, and RM-I071870 and RM-I0718755 overlap with RM-I071853. Only three sets of peaks were observed for the seven impurities.
The experimental results show that reducing the injection volume can achieve certain differentiation between the two structural signals RM-I071851 and RM-I071852, but the separation effect is not significant; And the RM-I071873/RM-I071800 signals are still indistinguishable, reminding researchers to pay attention to the use of analytical methods in the study of the above impurities. The liquid chromatography results and data of the mixed injection are shown in Figures 2 and 3:

Figure 2: Mixed Sample Figure 2
Data source: QCS Standard Material R&D Center

Figure 3: Summary of mixed injection data in Figure 2
Data source: QCS Standard Material R&D Center
In addition, it should be noted that the four impurities RM-I071870/RM-I0718755/RM-I071853/RM-I071873 cannot be effectively distinguished from other impurities under this standard chromatographic method. From Figure 1 and Figure 2, it can be seen that the three impurities RM-I071870/RM-I0718755/RM-I071853 cannot be distinguished from each other under standard chromatographic conditions, and RM-I071873 and the active pharmaceutical ingredient RM-I071800 cannot be distinguished. Using this chromatographic condition to study the above four impurities may require further optimization of the analytical method. The following is a summary of the chromatographic results of individual injections of RM-I071870, RM-I0718755, RM-I071853, RM-I071873, and the active pharmaceutical ingredient RM-I071800.



Figure 4: Five products individually sampled
Data source: QCS Standard Material R&D Center
The Iguratimod series impurities are a key variety developed by our company. After the full chain coordination of synthesis, analysis, and preparation by the company, the project took less than a month from the initial approval to the completion of the project. Since there is no relevant impurity information in the standard or literature, our company focuses on deducing the process and degradation impurities that may be generated in the process through in-depth study of the Iguratimod synthesis process. If you are interested in understanding how our company deduces and determines the above impurities, you can leave a comment on the official account. Later, we will make a special phase I of the ideas on the generation of all impurities and the pitfalls in the synthesis to give you a guide to avoid pitfalls.
Product Information
The summary table of product mixed injection data is shown in Figure 5:

Figure 5: Summary table of product mixed injection data
Data source: QCS Standard Material R&D Center

Figure 6: Structural information of related products
Data source: QCS Standard Material R&D Center
Long press to recognize the QR code and view the list of all impurities!

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号