Time:2023-05-30
Introduction: Today we will share the impurity research of a relatively niche drug - Ivabradine hydrochlorid. The reason for sharing this variety is mainly because its standard includes a large number of impurities, and the synthesis and analysis of these impurities are difficult. In the main text, we will provide a detailed explanation of the difficulties in the study of impurities in this series.
Ivabradine hydrochloride tablets (trade name:Corlentor) are used for NYHAII-IV grade chronic heart failure patients with sinus rhythm and heart rate ≥ 75 beats/minute, accompanied by cardiac systolic dysfunction. They are used in combination with standard therapy beta blockers or for the treatment of contraindications or intolerance to beta blockers. It is the first sinoatrial node If current selective specific inhibitor, and the 2012 European ESC heart failure guidelines clearly state that Ivabradine significantly improves the quality of life of heart failure patients.
At present, a total of 74 impurities of Ivabradine hydrochloride are included on the QCS official website (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the standard for Ivabradine hydrochloride tablets (standard number: JX20120088) and conducted liquid chromatography localization research on the impurities included in the code in the standard and those included in the USP official website (see Figure 6 for specific impurity structure formulas). The specific standard method and mixed injection liquid chromatography results are shown in Figure 1 and Figure 2:

Figure 1: Chromatographic conditions for import registration standards
Data source: QCS Standard Material R&D Center
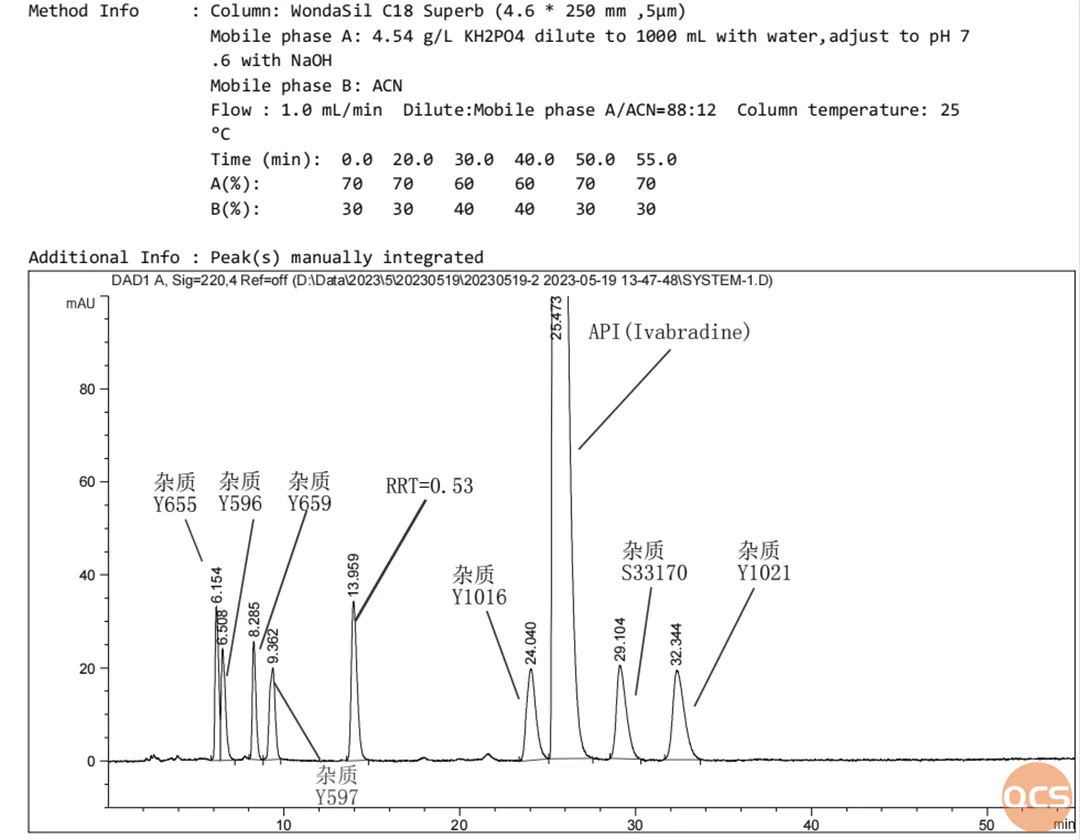
Figure 2: Sample mixture under standard chromatographic conditions
Data source: QCS Standard Material R&D Center
The above mixed injection data is based on the standard chromatogram (Figure 3) under the applicability method of the import registration standard system for reproducibility.
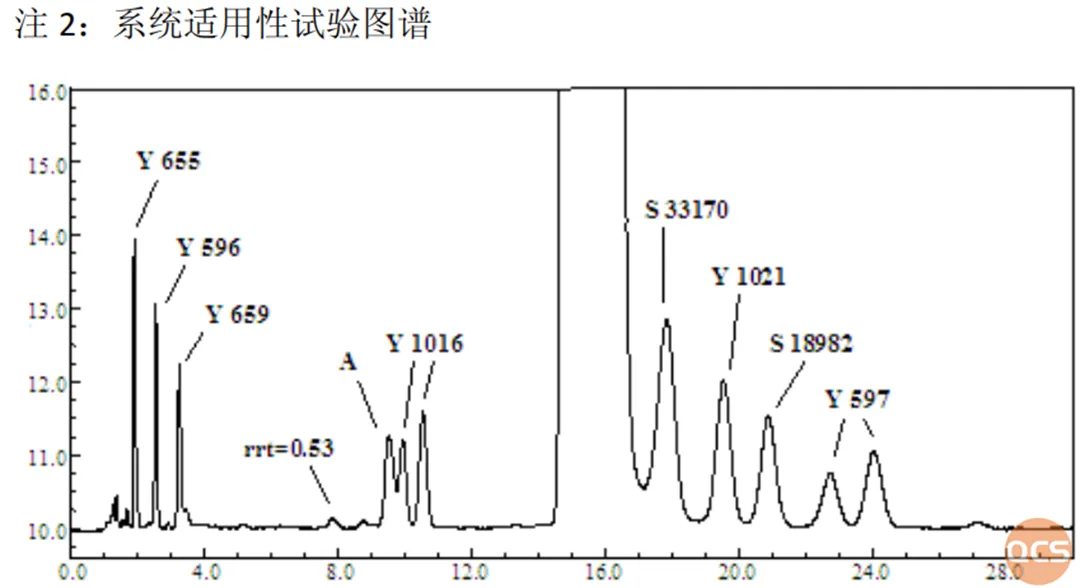
Figure 3: System applicability test diagram in the standard
Source: Import Registration Standard: JX20120088
From Figure 2 and Figure 3, most of the impurities are consistent with the system suitability spectrum in the standard, but it can also be seen that there are significant differences between Y1016 and the standard chromatogram. Due to the small difference in retention time among the three peaks of impurity A, impurity Y1016, and RRT=0.53, the central researcher mixed these three impurities with API and injected them into the sample for further confirmation. It can be seen that in further chromatographic validation, the above impurities have good separation efficiency, as shown in Figure 4:
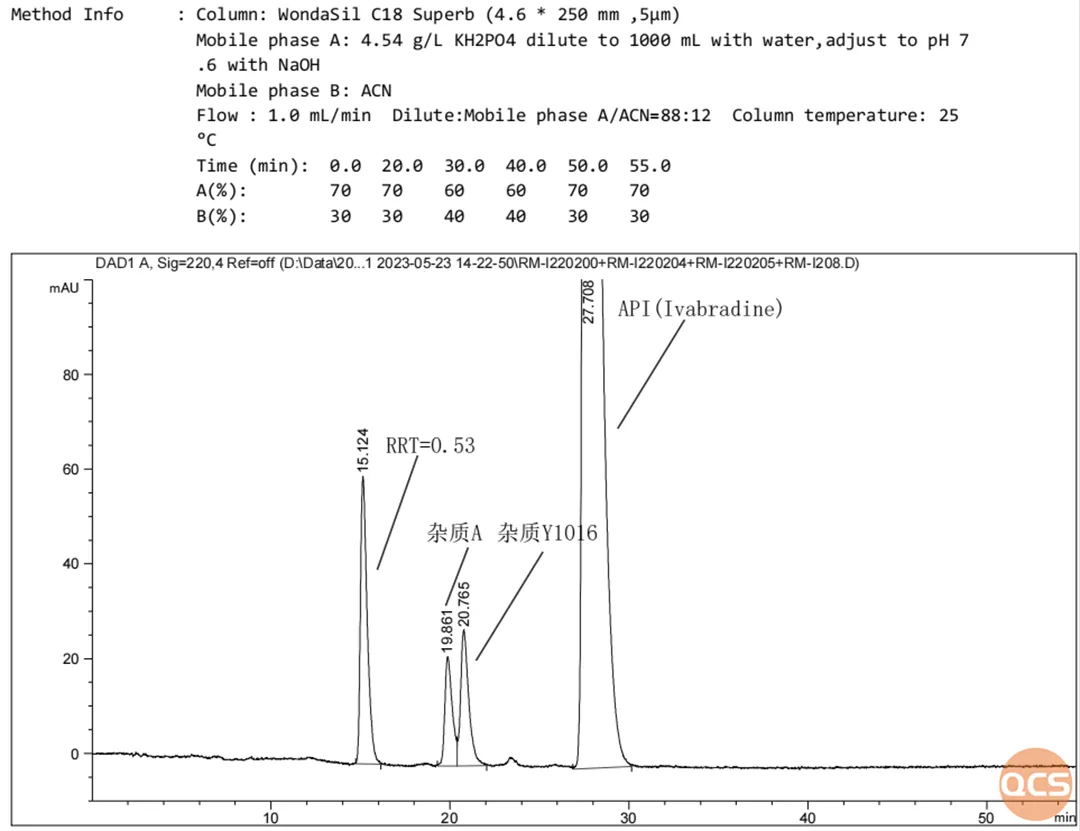
Figure 4: Impurity A, Impurity Y1016, and RRT=0.53 mixed into the sample with API
Data source: QCS Standard Material R&D Center
The corresponding relationship between import registration standards and impurities included in USP is now shared as follows (Figure 5)
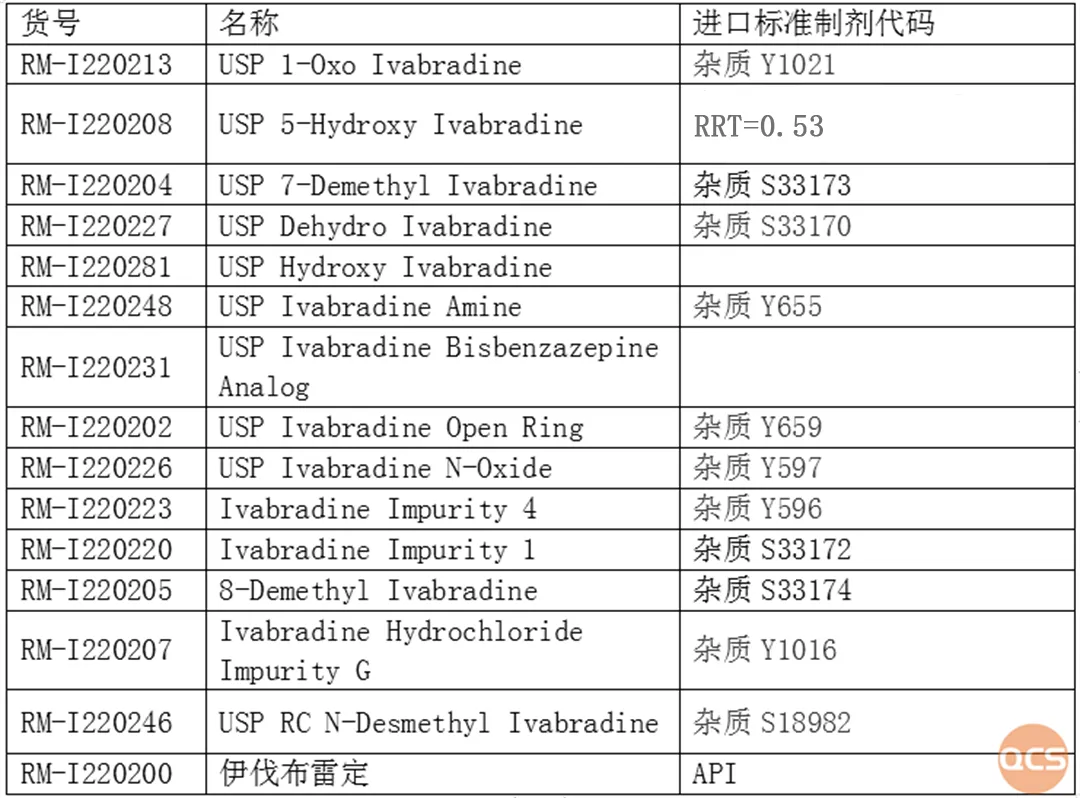
Figure 5: Correspondence between impurity codes and USP impurity names in import standards
Data source: QCS Standard Material R&D Center
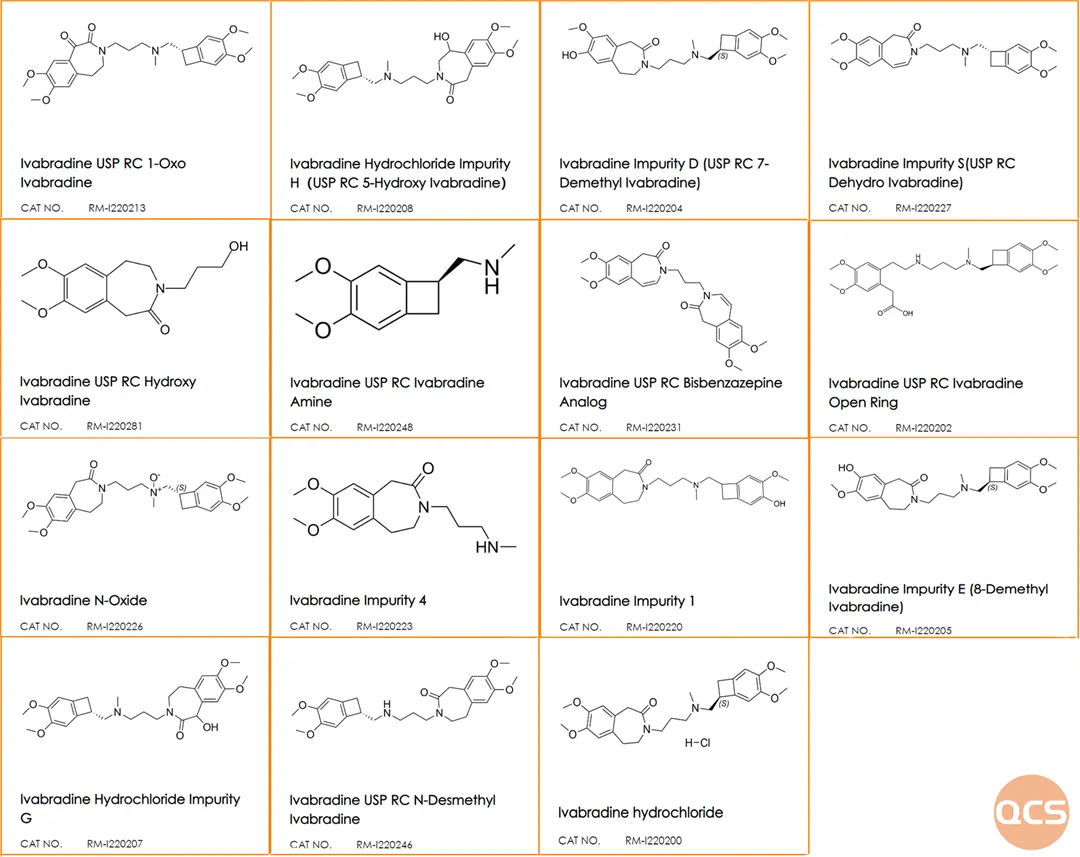
Figure 6: Structural information of related products
Data source: QCS Standard Material R&D Center
If you are a careful reader, you may have noticed that the Y1016 impurity in the import registration standard test results is bimodal. From the structural analysis, it can also be seen that the product is a mixture of diastereomers, and it is reasonable to have bimodal results in the reverse phase system. However, the chromatographic results of this study showed a single peak for Y1016 impurity (Figure 4)
We believe that the reason for this is due to differences in chromatographic columns. Due to the particularity of the chromatographic column (C16) in the import standard, the QCS R&D center selected a slightly different (WondaSil C18 Superb 4.6 * 250 mm, 5 μ m) chromatographic column for this study, which may be the reason for the differences. Further analysis of the impurity structure of Y1016 reveals that it is a mixture of diastereomers containing two chiral centers. However, the centers of Y1016's palms are far apart and it is an open chain molecule lacking rigidity. This type of diastereomer does not show significant differences under conventional chromatographic conditions, posing significant challenges to separation conditions. Due to differences in chromatographic columns, the current chromatographic conditions have poor discrimination of Y1016 diastereomers, and this chromatographic column has not been able to reproduce the results of the import registration standards well. Therefore, to address this issue, we further utilized chiral chromatography systems with better resolution of chiral isomers for further research.
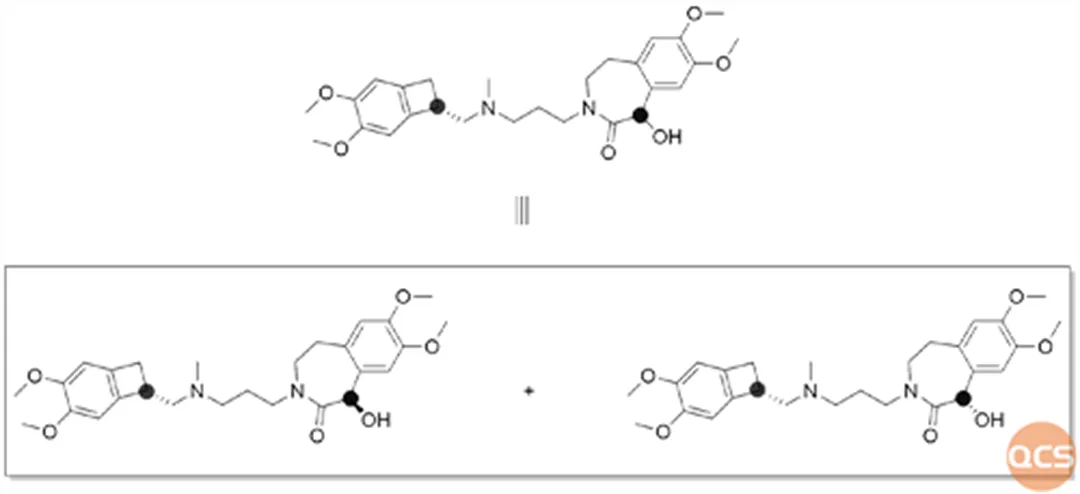
For impurity Y1016, the two chiral centers are chiral hydroxyl and chiral quaternary ring structures. The chiral quaternary ring structure is derived from commercially available optically pure (S) - configuration materials, while the hydroxyl unit is derived from the reduction of the ketone carbonyl group, resulting in a mixture of (R) - and (S) - chiral hydroxyl groups. Therefore, the R&D center used chiral chromatography columns that are more sensitive to chiral isomers to confirm it. It can be seen that the Y1016 impurity, which cannot be effectively distinguished under the original chromatographic conditions, exhibits good discrimination effect under chiral chromatographic conditions. The mass spectrometry results of each chromatographic peak are also consistent with the molecular weight. The specific hand detection data are shown in Figure 7:
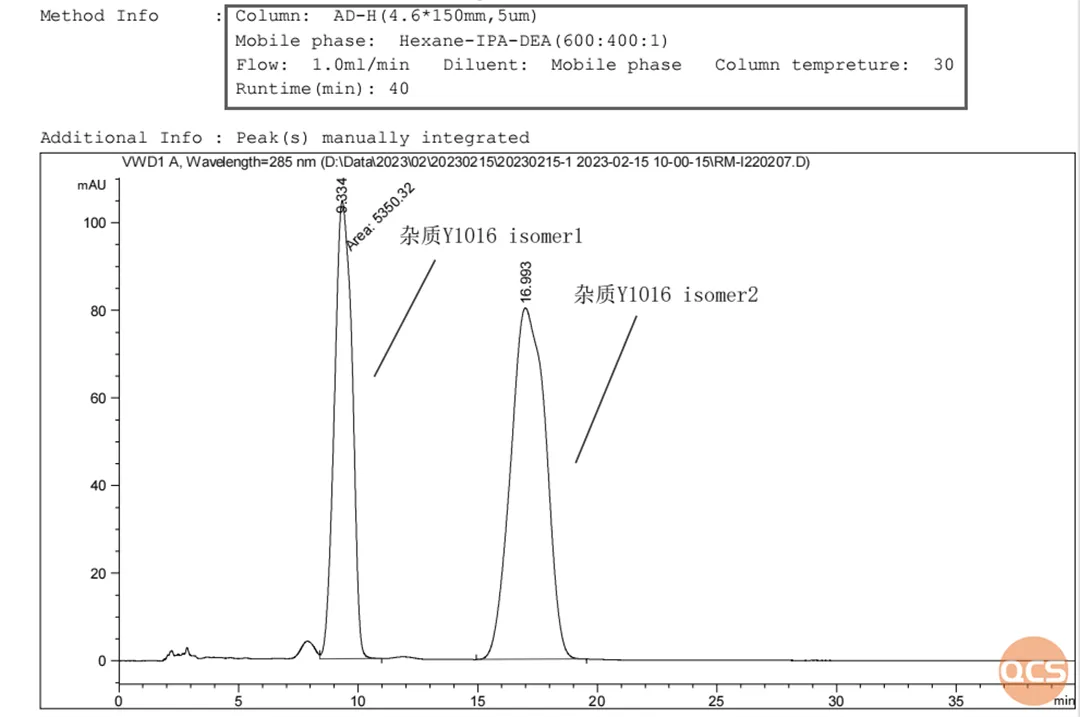
Figure 7: Chiral detection spectrum of impurity Y1016
Data source: QCS Standard Material R&D Center
The specific NMR and MS analysis data of impurity Y1016 are shown in Figure 8:
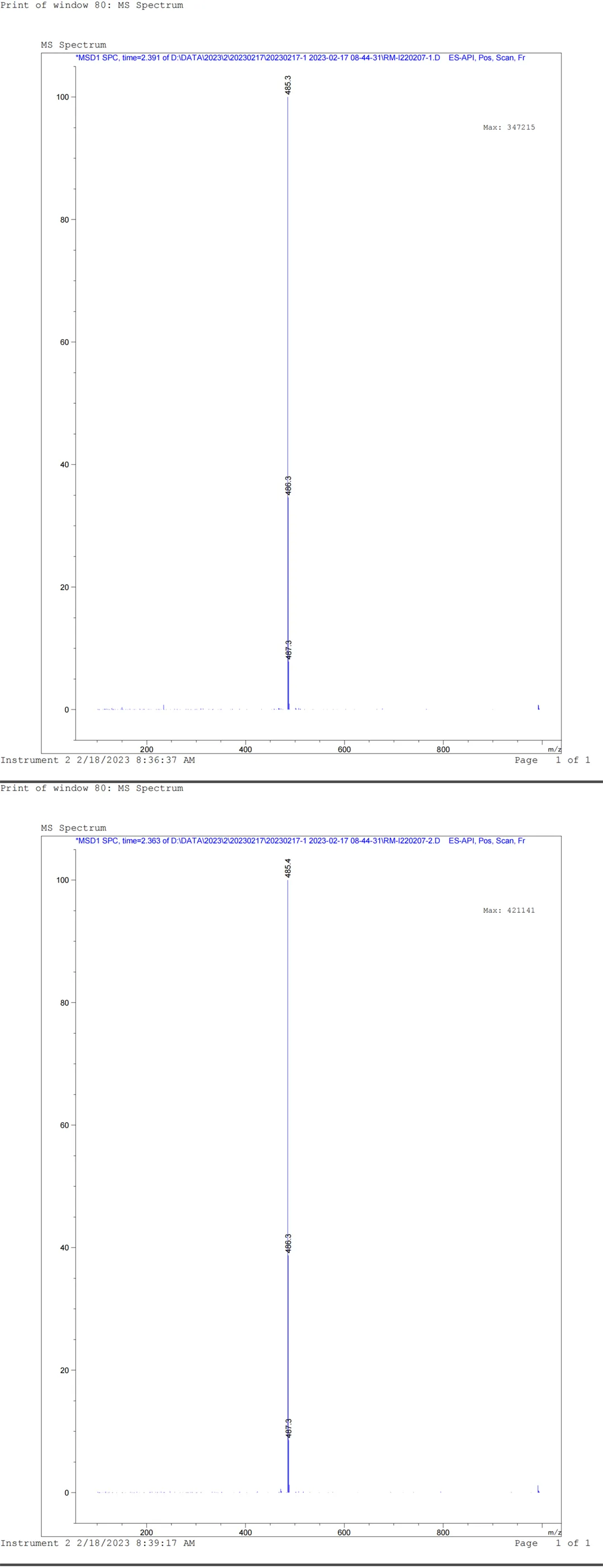
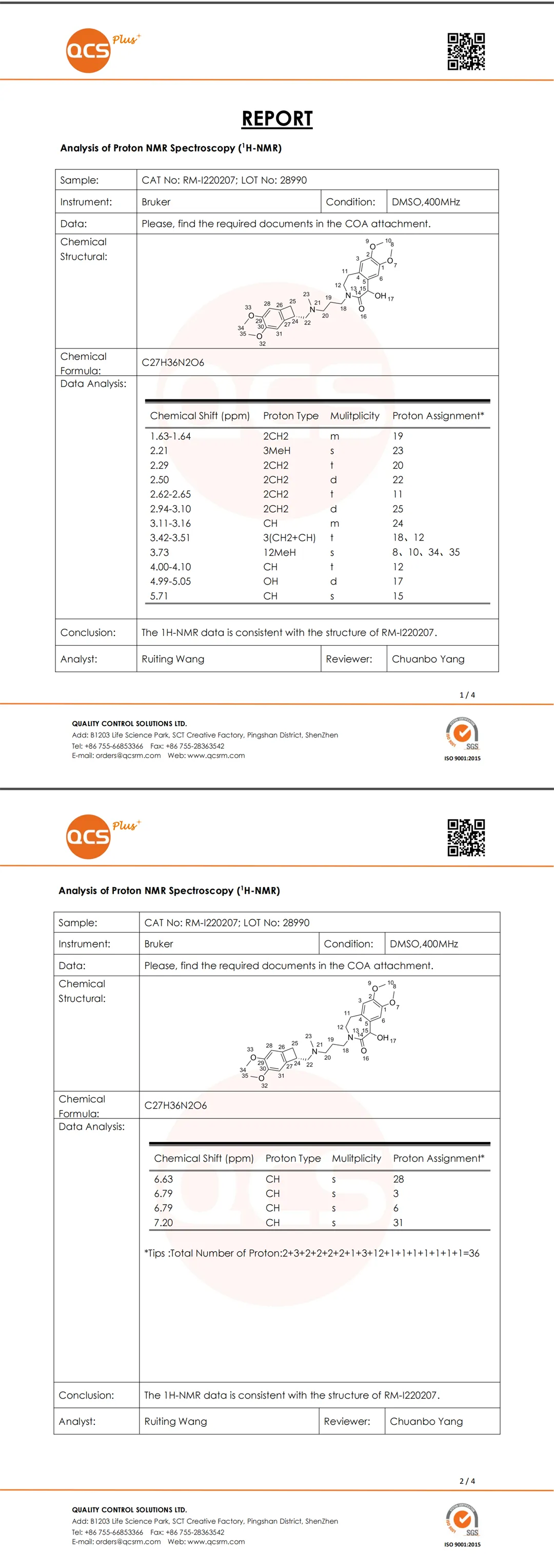

Figure 8: Structural analysis report of Impurity Y1016
Data source: QCS Standard Material R&D Center
Similar to the structure of impurity Y1016, Ivabradine has multiple impurities with different chiral center configurations that exhibit similar situations (such as Y597). Due to differences in conventional chromatography columns and chromatographic conditions, non enantiomeric mixtures lacking rigid structures or components with minimal structural differences may have unclear distinctions. It is recommended to use chiral chromatography columns for research. The QCS R&D center is equipped with chiral detection equipment and various types of chiral chromatography columns, which can meet the needs of chiral research. If any customers encounter similar problems, they can discuss and communicate with us, and let's work together to solve the problems in drug production and research. Strictly control the quality, so that the people can use good medicine with peace of mind.
Long press to recognize the QR code and view the list of all impurities!


Introduction: Today we will share the impurity research of a relatively niche drug - Ivabradine hydrochlorid. The reason for sharing this variety is mainly because its standard includes a large number of impurities, and the synthesis and analysis of these impurities are difficult. In the main text, we will provide a detailed explanation of the difficulties in the study of impurities in this series.
Ivabradine hydrochloride tablets (trade name:Corlentor) are used for NYHAII-IV grade chronic heart failure patients with sinus rhythm and heart rate ≥ 75 beats/minute, accompanied by cardiac systolic dysfunction. They are used in combination with standard therapy beta blockers or for the treatment of contraindications or intolerance to beta blockers. It is the first sinoatrial node If current selective specific inhibitor, and the 2012 European ESC heart failure guidelines clearly state that Ivabradine significantly improves the quality of life of heart failure patients.
At present, a total of 74 impurities of Ivabradine hydrochloride are included on the QCS official website (scan the QR code at the end of the article to view the list of all impurities). Our center referred to the standard for Ivabradine hydrochloride tablets (standard number: JX20120088) and conducted liquid chromatography localization research on the impurities included in the code in the standard and those included in the USP official website (see Figure 6 for specific impurity structure formulas). The specific standard method and mixed injection liquid chromatography results are shown in Figure 1 and Figure 2:

Figure 1: Chromatographic conditions for import registration standards
Data source: QCS Standard Material R&D Center

Figure 2: Sample mixture under standard chromatographic conditions
Data source: QCS Standard Material R&D Center
The above mixed injection data is based on the standard chromatogram (Figure 3) under the applicability method of the import registration standard system for reproducibility.

Figure 3: System applicability test diagram in the standard
Source: Import Registration Standard: JX20120088
From Figure 2 and Figure 3, most of the impurities are consistent with the system suitability spectrum in the standard, but it can also be seen that there are significant differences between Y1016 and the standard chromatogram. Due to the small difference in retention time among the three peaks of impurity A, impurity Y1016, and RRT=0.53, the central researcher mixed these three impurities with API and injected them into the sample for further confirmation. It can be seen that in further chromatographic validation, the above impurities have good separation efficiency, as shown in Figure 4:

Figure 4: Impurity A, Impurity Y1016, and RRT=0.53 mixed into the sample with API
Data source: QCS Standard Material R&D Center
The corresponding relationship between import registration standards and impurities included in USP is now shared as follows (Figure 5)

Figure 5: Correspondence between impurity codes and USP impurity names in import standards
Data source: QCS Standard Material R&D Center

Figure 6: Structural information of related products
Data source: QCS Standard Material R&D Center
If you are a careful reader, you may have noticed that the Y1016 impurity in the import registration standard test results is bimodal. From the structural analysis, it can also be seen that the product is a mixture of diastereomers, and it is reasonable to have bimodal results in the reverse phase system. However, the chromatographic results of this study showed a single peak for Y1016 impurity (Figure 4)
We believe that the reason for this is due to differences in chromatographic columns. Due to the particularity of the chromatographic column (C16) in the import standard, the QCS R&D center selected a slightly different (WondaSil C18 Superb 4.6 * 250 mm, 5 μ m) chromatographic column for this study, which may be the reason for the differences. Further analysis of the impurity structure of Y1016 reveals that it is a mixture of diastereomers containing two chiral centers. However, the centers of Y1016's palms are far apart and it is an open chain molecule lacking rigidity. This type of diastereomer does not show significant differences under conventional chromatographic conditions, posing significant challenges to separation conditions. Due to differences in chromatographic columns, the current chromatographic conditions have poor discrimination of Y1016 diastereomers, and this chromatographic column has not been able to reproduce the results of the import registration standards well. Therefore, to address this issue, we further utilized chiral chromatography systems with better resolution of chiral isomers for further research.

For impurity Y1016, the two chiral centers are chiral hydroxyl and chiral quaternary ring structures. The chiral quaternary ring structure is derived from commercially available optically pure (S) - configuration materials, while the hydroxyl unit is derived from the reduction of the ketone carbonyl group, resulting in a mixture of (R) - and (S) - chiral hydroxyl groups. Therefore, the R&D center used chiral chromatography columns that are more sensitive to chiral isomers to confirm it. It can be seen that the Y1016 impurity, which cannot be effectively distinguished under the original chromatographic conditions, exhibits good discrimination effect under chiral chromatographic conditions. The mass spectrometry results of each chromatographic peak are also consistent with the molecular weight. The specific hand detection data are shown in Figure 7:

Figure 7: Chiral detection spectrum of impurity Y1016
Data source: QCS Standard Material R&D Center
The specific NMR and MS analysis data of impurity Y1016 are shown in Figure 8:



Figure 8: Structural analysis report of Impurity Y1016
Data source: QCS Standard Material R&D Center
Similar to the structure of impurity Y1016, Ivabradine has multiple impurities with different chiral center configurations that exhibit similar situations (such as Y597). Due to differences in conventional chromatography columns and chromatographic conditions, non enantiomeric mixtures lacking rigid structures or components with minimal structural differences may have unclear distinctions. It is recommended to use chiral chromatography columns for research. The QCS R&D center is equipped with chiral detection equipment and various types of chiral chromatography columns, which can meet the needs of chiral research. If any customers encounter similar problems, they can discuss and communicate with us, and let's work together to solve the problems in drug production and research. Strictly control the quality, so that the people can use good medicine with peace of mind.
Long press to recognize the QR code and view the list of all impurities!


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号