Time:2023-06-01
As per the latest announcement on the official website of the CDE, the application for generic listing of Rucotinib phosphate tablets, a Class 4 chemical drug developed by Chengdu Yuandong Biological, has been accepted. In 2022, global sales of JAK inhibitors reached $4 billion, with domestic sales experiencing a year-on-year increase of 23%, totaling $400 million. Currently, two pharmaceutical companies—Nanjing Zhengda Tianqing and Chengdu Yuandong Biological—have submitted applications for generic drug listings for this product, vying to be the first domestic manufacturer to produce a JAK inhibitor.
Ruxolitinib Phosphate (trade name: Jakavi) is an antitumor agent indicated for adults with moderate to high-risk primary myelofibrosis (PMF), also referred to as chronic idiopathic myelofibrosis, as well as myelofibrosis secondary to polycythemia vera (PPV-MF) or primary thrombocythemia (PET-MF). It is utilized in the management of splenomegaly associated with these conditions or related symptomatic manifestations.
Currently, a total of 31 impurities related to Rucotinib phosphate are listed on the official QCS website (please scan the QR code at the end of this article to access the complete list). Our center adheres to the preparation standard for Rucotinib phosphate tablets (standard number: JX20140057), wherein the coded impurities specified in this standard were identified through liquid chromatography and system suitability studies (API calibration). The detailed standard methodology and results from mixed injection liquid chromatography are presented in FIG. 1 and FIG. 3.
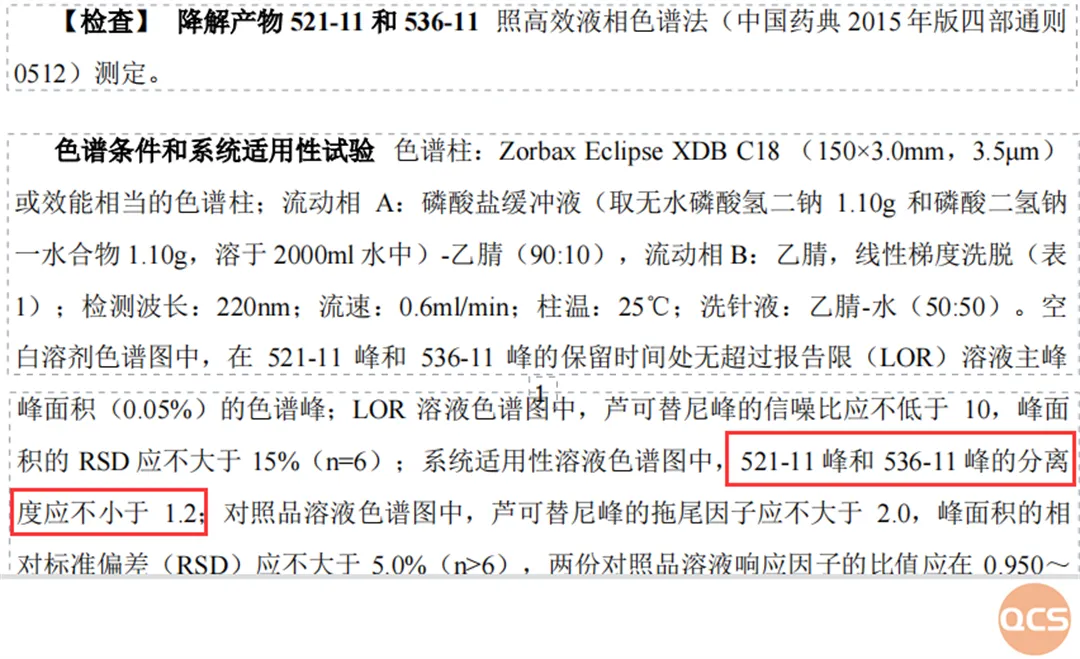
Figure 1: Standard chromatographic parameters for import registration
Data source: QCS Standard Materials Research and Development Center
In this standard, three related substances are designated as 521-11 (RM-R241207), 536-11 (RM-R241210), and 518-11 (RM-R241201), with 518-11 identified as an enantiomeric impurity of lucotinib. The QCS R&D center conducted a comprehensive study on this series of compounds, presenting the detection results for the aforementioned impurities under both reversed-phase chromatography and chiral chromatography conditions for reference.
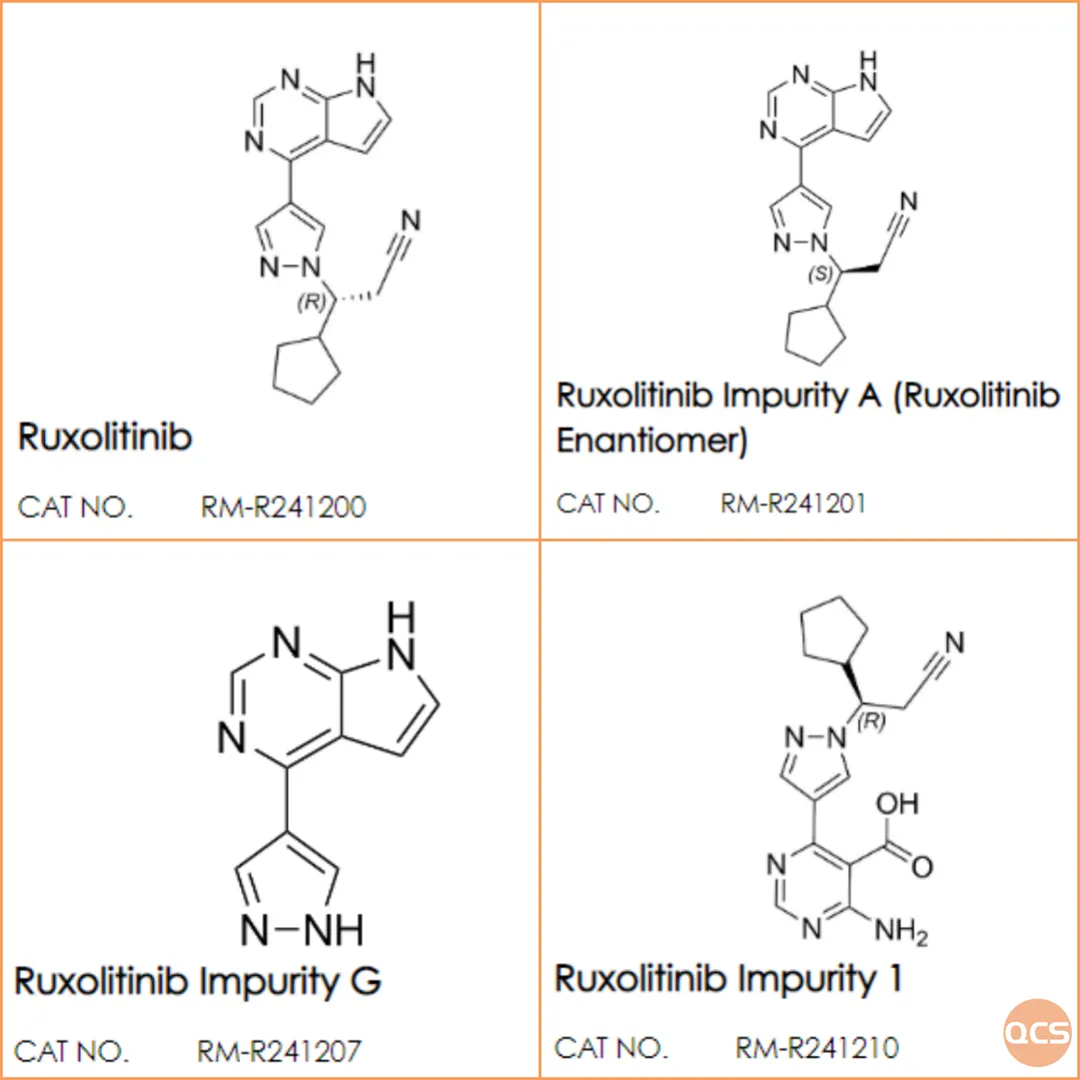
Figure 2: Pertinent structural information regarding the product
Data Source: QCS Standard Materials Research and Development Center
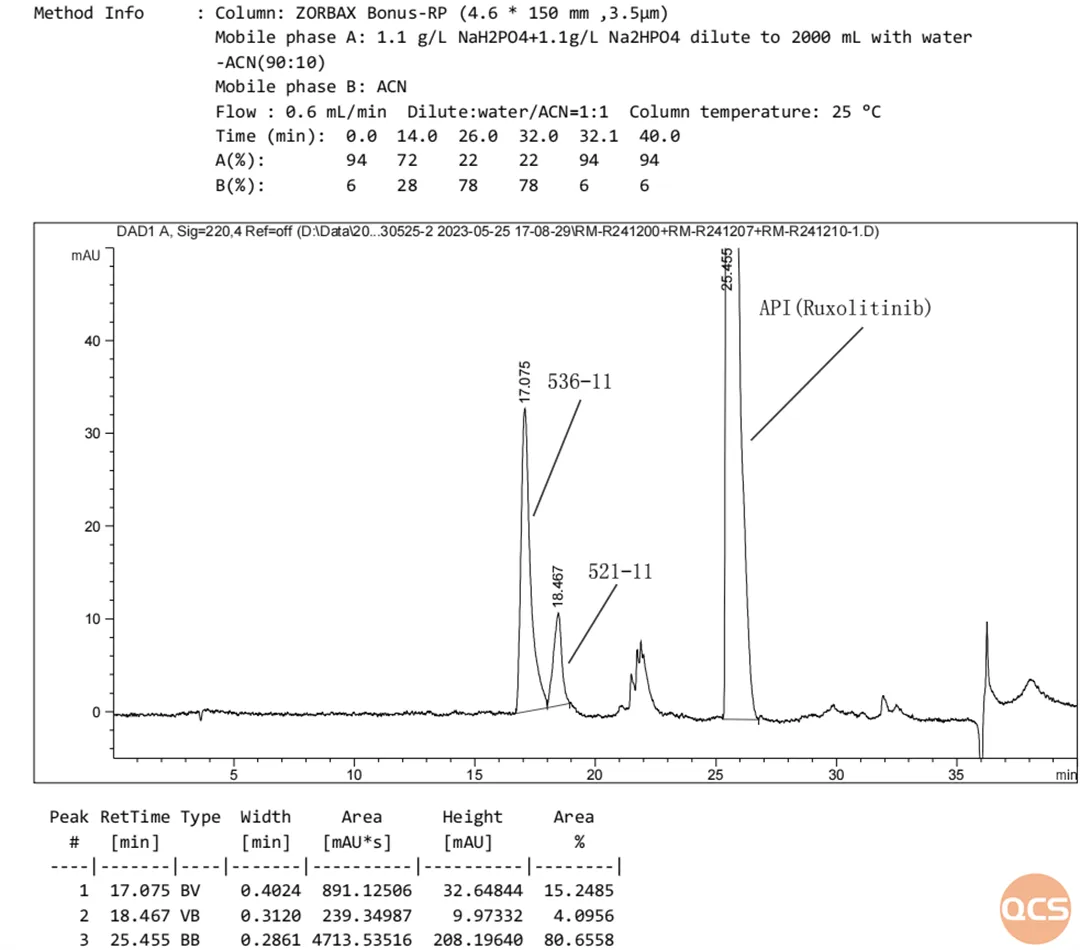
Figure 3: Sample Mixture Analyzed under Standard Chromatographic Conditions
Data Source: QCS Standard Materials Research and Development Center
Initially, we refer to the chapter on the detection of relevant substances in the import registration standard, which stipulates that the separation degree between code impurities 536-11 and 521-11 must not be less than 1.2 under reverse phase chromatography conditions. Through column optimization, we achieved a separation factor of 1.99 for these target impurities using a ZORBAX Bonus-RP column (4.6 × 150 mm, 3.5 μm). Consequently, our center adopted this condition for subsequent work to calibrate the contents of rucotinib and rucotinib phosphate individually.
During the research process, we identified certain challenges regarding the stability of the solution under evaluation. Our R&D team discovered that the duration of sample placement significantly influenced test results during calibration attempts. The data obtained from this sample demonstrated good repeatability under current conditions. However, if there is a substantial deviation in detection data after prolonged placement, the recovery rate may be deemed unsatisfactory. We advise customers to pay closer attention to product stability when utilizing the API reference.
Following the completion of our study into the conditions of conventional reversed-phase chromatography, our center undertook a specialized study focusing on the enantiomeric impurities within the Lucotinib impurity series. In conjunction with the relevant isomer inspection criteria outlined in import registration standards, we employed these conditions to examine the Rucotinib hand-type impurity (RM-R241201) and compiled a comprehensive summary of both this impurity and its active pharmaceutical ingredient (API) under these chromatographic parameters. The methodologies and findings are illustrated in FIG. 4 and FIG. 5.
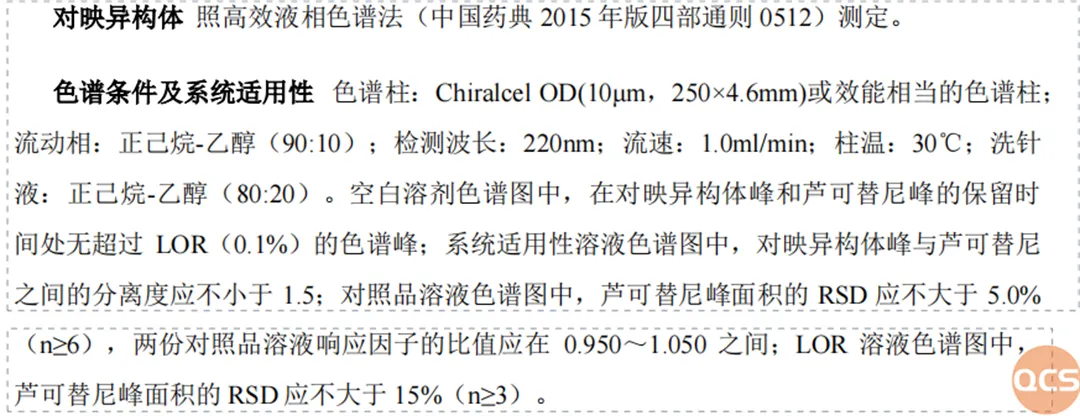
Figure 4: Conditions for the detection of Lucotinib's corresponding isomers
Data Source: QCS Standard Materials Research and Development Center
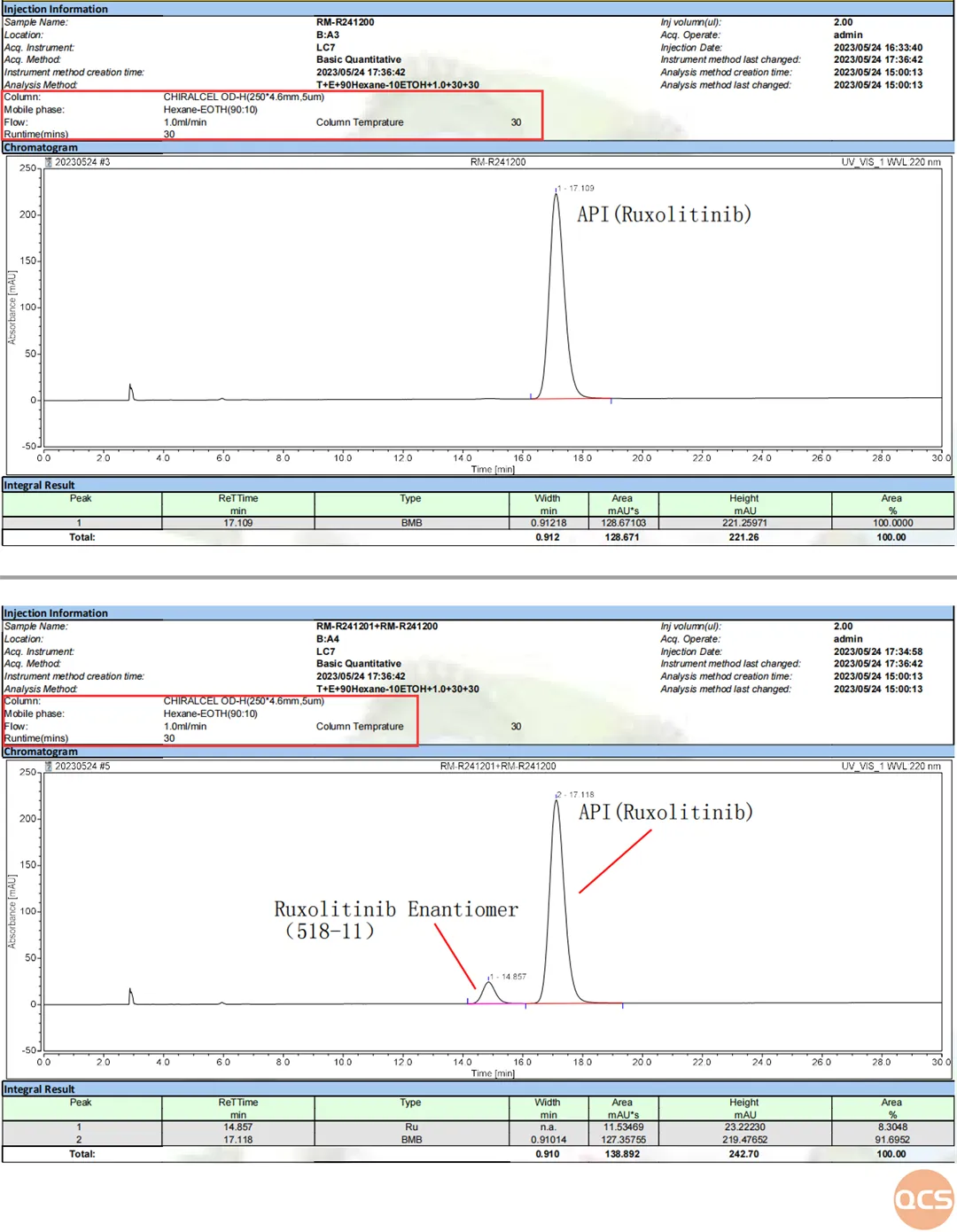
Figure 5: Rucotinib administered alone and in combination with its corresponding isomers
Data Source: QCS Standard Materials Research and Development Center
The mixed sample data of Rucotinib API and the enantiomer impurity RM-R241201, as illustrated in the figure above, demonstrates a commendable separation between Rucotinib and its corresponding isomers, with both peaks achieving excellent resolution. Given the heightened scrutiny regarding the corresponding isomers of APIs during drug review processes, employing high-grade standard samples will facilitate the acquisition of more reliable test data.
In light of the growing demand for chiral research within the pharmaceutical development process, the QCS Standard Material Research Center is committed to providing high-quality standard material samples to fulfill your chiral testing requirements. The QCS R&D Center is equipped with advanced chiral testing apparatus and a variety of chiral columns, enabling us to address the needs of drug chirality research effectively and assist you in overcoming challenges in drug production and development. Let us collaborate closely to ensure stringent quality control, ensuring that effective medications are accessible to all.
Press and hold the identification QR code to access a comprehensive list of all impurities


As per the latest announcement on the official website of the CDE, the application for generic listing of Rucotinib phosphate tablets, a Class 4 chemical drug developed by Chengdu Yuandong Biological, has been accepted. In 2022, global sales of JAK inhibitors reached $4 billion, with domestic sales experiencing a year-on-year increase of 23%, totaling $400 million. Currently, two pharmaceutical companies—Nanjing Zhengda Tianqing and Chengdu Yuandong Biological—have submitted applications for generic drug listings for this product, vying to be the first domestic manufacturer to produce a JAK inhibitor.
Ruxolitinib Phosphate (trade name: Jakavi) is an antitumor agent indicated for adults with moderate to high-risk primary myelofibrosis (PMF), also referred to as chronic idiopathic myelofibrosis, as well as myelofibrosis secondary to polycythemia vera (PPV-MF) or primary thrombocythemia (PET-MF). It is utilized in the management of splenomegaly associated with these conditions or related symptomatic manifestations.
Currently, a total of 31 impurities related to Rucotinib phosphate are listed on the official QCS website (please scan the QR code at the end of this article to access the complete list). Our center adheres to the preparation standard for Rucotinib phosphate tablets (standard number: JX20140057), wherein the coded impurities specified in this standard were identified through liquid chromatography and system suitability studies (API calibration). The detailed standard methodology and results from mixed injection liquid chromatography are presented in FIG. 1 and FIG. 3.

Figure 1: Standard chromatographic parameters for import registration
Data source: QCS Standard Materials Research and Development Center
In this standard, three related substances are designated as 521-11 (RM-R241207), 536-11 (RM-R241210), and 518-11 (RM-R241201), with 518-11 identified as an enantiomeric impurity of lucotinib. The QCS R&D center conducted a comprehensive study on this series of compounds, presenting the detection results for the aforementioned impurities under both reversed-phase chromatography and chiral chromatography conditions for reference.

Figure 2: Pertinent structural information regarding the product
Data Source: QCS Standard Materials Research and Development Center

Figure 3: Sample Mixture Analyzed under Standard Chromatographic Conditions
Data Source: QCS Standard Materials Research and Development Center
Initially, we refer to the chapter on the detection of relevant substances in the import registration standard, which stipulates that the separation degree between code impurities 536-11 and 521-11 must not be less than 1.2 under reverse phase chromatography conditions. Through column optimization, we achieved a separation factor of 1.99 for these target impurities using a ZORBAX Bonus-RP column (4.6 × 150 mm, 3.5 μm). Consequently, our center adopted this condition for subsequent work to calibrate the contents of rucotinib and rucotinib phosphate individually.
During the research process, we identified certain challenges regarding the stability of the solution under evaluation. Our R&D team discovered that the duration of sample placement significantly influenced test results during calibration attempts. The data obtained from this sample demonstrated good repeatability under current conditions. However, if there is a substantial deviation in detection data after prolonged placement, the recovery rate may be deemed unsatisfactory. We advise customers to pay closer attention to product stability when utilizing the API reference.
Following the completion of our study into the conditions of conventional reversed-phase chromatography, our center undertook a specialized study focusing on the enantiomeric impurities within the Lucotinib impurity series. In conjunction with the relevant isomer inspection criteria outlined in import registration standards, we employed these conditions to examine the Rucotinib hand-type impurity (RM-R241201) and compiled a comprehensive summary of both this impurity and its active pharmaceutical ingredient (API) under these chromatographic parameters. The methodologies and findings are illustrated in FIG. 4 and FIG. 5.

Figure 4: Conditions for the detection of Lucotinib's corresponding isomers
Data Source: QCS Standard Materials Research and Development Center

Figure 5: Rucotinib administered alone and in combination with its corresponding isomers
Data Source: QCS Standard Materials Research and Development Center
The mixed sample data of Rucotinib API and the enantiomer impurity RM-R241201, as illustrated in the figure above, demonstrates a commendable separation between Rucotinib and its corresponding isomers, with both peaks achieving excellent resolution. Given the heightened scrutiny regarding the corresponding isomers of APIs during drug review processes, employing high-grade standard samples will facilitate the acquisition of more reliable test data.
In light of the growing demand for chiral research within the pharmaceutical development process, the QCS Standard Material Research Center is committed to providing high-quality standard material samples to fulfill your chiral testing requirements. The QCS R&D Center is equipped with advanced chiral testing apparatus and a variety of chiral columns, enabling us to address the needs of drug chirality research effectively and assist you in overcoming challenges in drug production and development. Let us collaborate closely to ensure stringent quality control, ensuring that effective medications are accessible to all.
Press and hold the identification QR code to access a comprehensive list of all impurities


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号