Time:2024-11-01
Introduction
In this study, we present an analysis of the stability of specific impurities in Rivaroxaban, a highly selective and dose-dependent novel oral anticoagulant. This agent exerts its anticoagulant effects by inhibiting both endogenous and exogenous pathways of Factor Xa (FXa), thereby disrupting the coagulation cascade and preventing thrombin production and thrombosis.
Experimental Scheme
In this study, our center conducted a study into the solution stability of three specific impurities in Rivaroxaban, utilizing chromatographic conditions outlined in the 'Related Substances' inspection criteria as per the import drug registration standard for Rivaroxaban Tablets (Standard No. YBH04672023). The sample numbers and structural formulas employed are illustrated in FIG. 1 and FIG. 2.
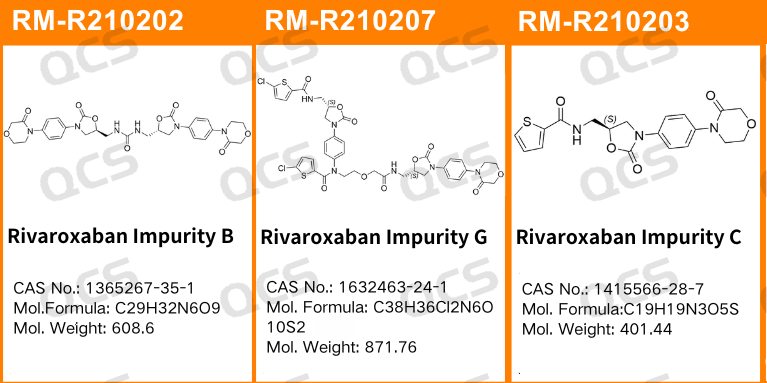
Figure 1: The impurity CAT numbers and structural formulas utilized in this study
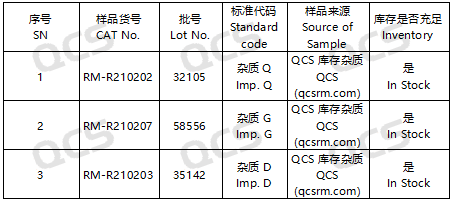
Figure 2: The correlation between the standard codes specified in the standard and the impurity item numbers employed in this study
In this experiment, the researcher utilized RM-R210202 (Impurity Q; Rivaroxaban Impurity B; CAS No: 1365267-35-1), RM-R210203 (Impurity D; Rivaroxaban Impurity C; CAS No: 1415566-28-7), and RM-R210207 (Impurity G; Rivaroxaban Impurity G; CAS No: 1632463-24-1). Appropriate quantities of these substances were placed in acidic, neutral, and alkaline solutions at room temperature and atmospheric pressure for durations of 0, 3, 6, 12, and 24 hours. This was conducted in accordance with the chromatographic conditions specified in the "Related Substances" section of the import drug registration standard for Rivaroxaban Tablets (Standard No. YBH04672023). The variation in the main peak area observed on the chromatogram was monitored as a function of storage time for each sample solution. Based on these observations, the stability of each sample solution was assessed.
Experimental Conclusions
Upon conducting tests, it was observed that the peak-to-peak areas of samples RM-R210202 (impurity Q), RM-R210203 (impurity D), and RM-R210207 (impurity G) exhibited minimal variation over a 24-hour period when immersed in acidic, neutral, and alkaline solutions. The relative standard deviations for all samples were consistently below 2.0%. Consequently, it can be inferred that these three samples demonstrate relative stability when subjected to acidic, neutral, and alkaline environments for 24 hours. The data regarding the main peak areas of samples RM-R210202 (impurity Q), RM-R210203 (impurity D), and RM-R210207 (impurity G) at various pH levels during each detection point is presented as follows:
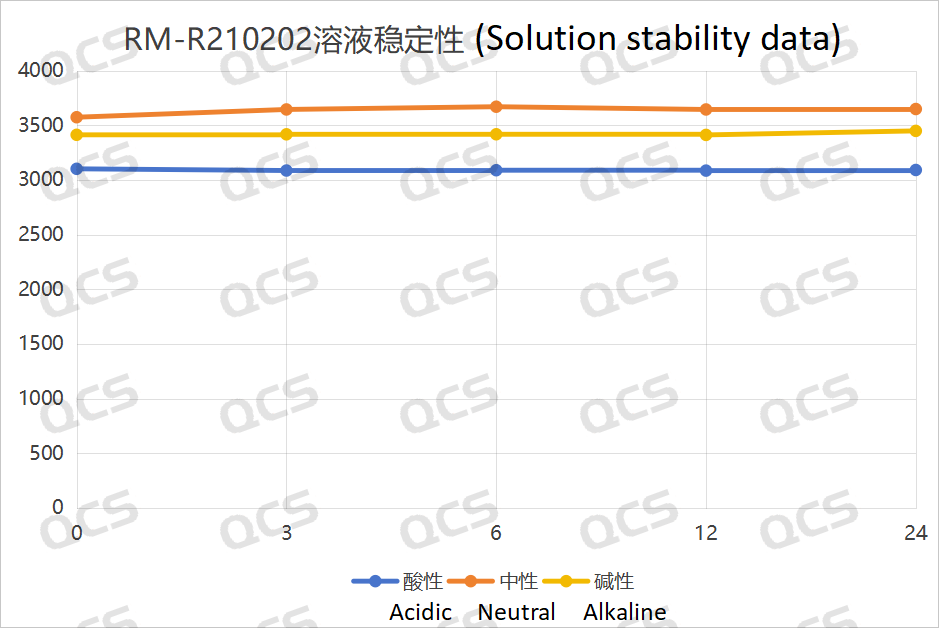
Figure 3: Line diagram summarizing the solution stability data for sample RM-R210202 (impurity Q)
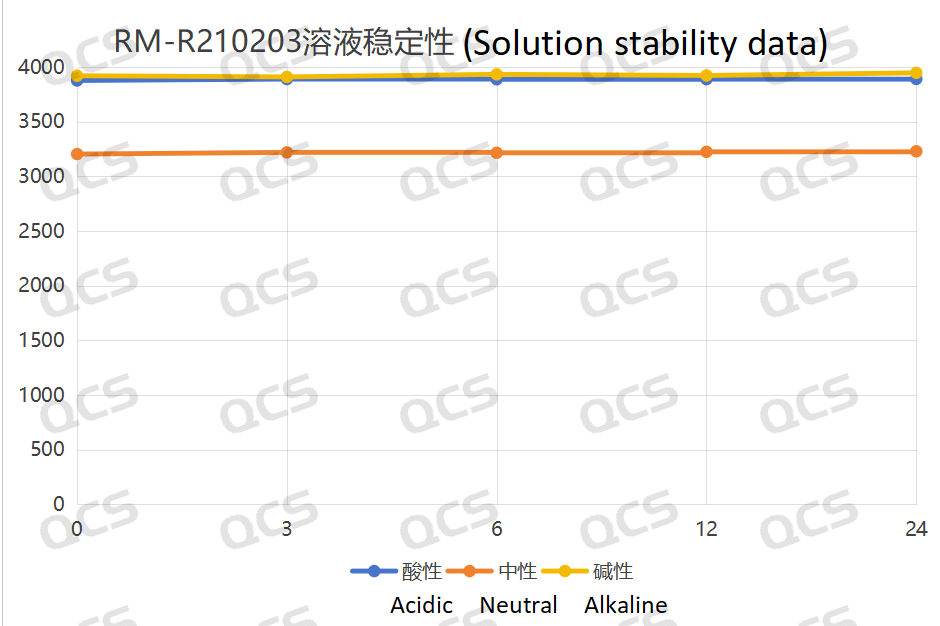
Figure 4: Line diagram summarizing the solution stability data for sample RM-R210203 (impurity D)
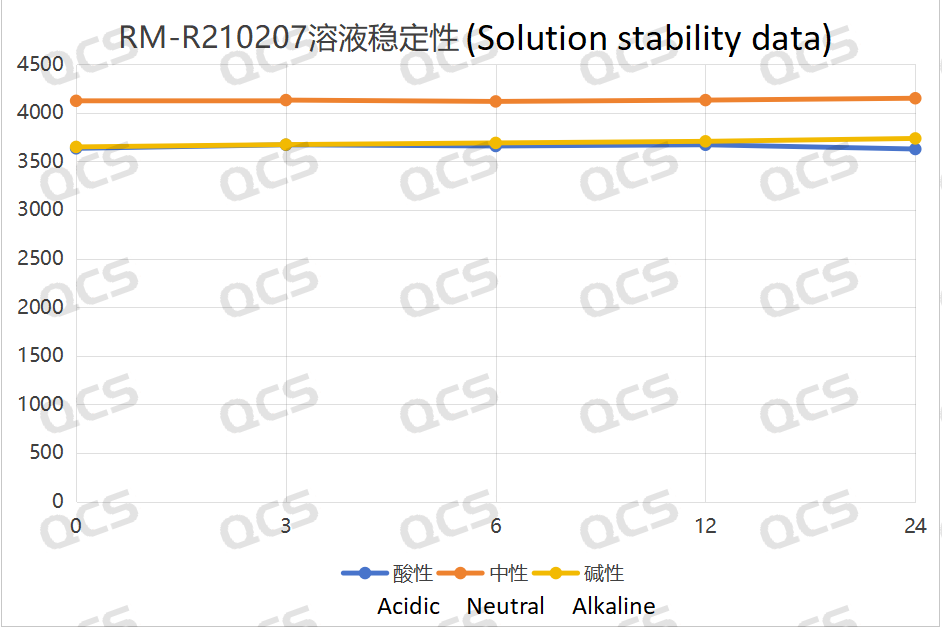
Figure 5: Line diagram summarizing the solution stability data for sample RM-R210207 (impurity G)
Summary
In conclusion, our experimental findings reveal that samples RM-R210202 (impurity Q), RM-R210203 (impurity D), and RM-R210207 (impurity G) demonstrate remarkable stability across a spectrum of acidic, alkaline, and neutral solutions. Should the esteemed customer seek comprehensive insights into the stability characteristics of these three samples, we warmly invite them to engage in consultation with our company.


Introduction
In this study, we present an analysis of the stability of specific impurities in Rivaroxaban, a highly selective and dose-dependent novel oral anticoagulant. This agent exerts its anticoagulant effects by inhibiting both endogenous and exogenous pathways of Factor Xa (FXa), thereby disrupting the coagulation cascade and preventing thrombin production and thrombosis.
Experimental Scheme
In this study, our center conducted a study into the solution stability of three specific impurities in Rivaroxaban, utilizing chromatographic conditions outlined in the 'Related Substances' inspection criteria as per the import drug registration standard for Rivaroxaban Tablets (Standard No. YBH04672023). The sample numbers and structural formulas employed are illustrated in FIG. 1 and FIG. 2.

Figure 1: The impurity CAT numbers and structural formulas utilized in this study

Figure 2: The correlation between the standard codes specified in the standard and the impurity item numbers employed in this study
In this experiment, the researcher utilized RM-R210202 (Impurity Q; Rivaroxaban Impurity B; CAS No: 1365267-35-1), RM-R210203 (Impurity D; Rivaroxaban Impurity C; CAS No: 1415566-28-7), and RM-R210207 (Impurity G; Rivaroxaban Impurity G; CAS No: 1632463-24-1). Appropriate quantities of these substances were placed in acidic, neutral, and alkaline solutions at room temperature and atmospheric pressure for durations of 0, 3, 6, 12, and 24 hours. This was conducted in accordance with the chromatographic conditions specified in the "Related Substances" section of the import drug registration standard for Rivaroxaban Tablets (Standard No. YBH04672023). The variation in the main peak area observed on the chromatogram was monitored as a function of storage time for each sample solution. Based on these observations, the stability of each sample solution was assessed.
Experimental Conclusions
Upon conducting tests, it was observed that the peak-to-peak areas of samples RM-R210202 (impurity Q), RM-R210203 (impurity D), and RM-R210207 (impurity G) exhibited minimal variation over a 24-hour period when immersed in acidic, neutral, and alkaline solutions. The relative standard deviations for all samples were consistently below 2.0%. Consequently, it can be inferred that these three samples demonstrate relative stability when subjected to acidic, neutral, and alkaline environments for 24 hours. The data regarding the main peak areas of samples RM-R210202 (impurity Q), RM-R210203 (impurity D), and RM-R210207 (impurity G) at various pH levels during each detection point is presented as follows:

Figure 3: Line diagram summarizing the solution stability data for sample RM-R210202 (impurity Q)

Figure 4: Line diagram summarizing the solution stability data for sample RM-R210203 (impurity D)

Figure 5: Line diagram summarizing the solution stability data for sample RM-R210207 (impurity G)
Summary
In conclusion, our experimental findings reveal that samples RM-R210202 (impurity Q), RM-R210203 (impurity D), and RM-R210207 (impurity G) demonstrate remarkable stability across a spectrum of acidic, alkaline, and neutral solutions. Should the esteemed customer seek comprehensive insights into the stability characteristics of these three samples, we warmly invite them to engage in consultation with our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号