Time:2024-03-28
Introduction:
Today, we present a relatively ‘popolar’ drug—Creborol ointment—and its related impurity research. However, data from the Drug Intelligence Network indicates that only the original research manufacturer, Pfizer, has been approved for listing in China, with merely three generic pharmaceutical companies (Hangzhou Industry, Qilu Pharmaceutical, and Nanjing Wanrong Jiancheng & Jiangsu Wanhe) having submitted applications for acceptance. Nevertheless, internal data from Zhenqiang Biological reveal that there are currently over 20 varieties under investigation within research enterprises. This suggests a highly competitive landscape in terms of imitation.
Creborol ointment, a phosphodiesterase 4 inhibitor, is indicated for the topical management of mild to moderate atopic dermatitis in patients aged 2 years and older. In December 2016, Creborol ointment received approval from the US FDA under the trade name Eucrisa and was subsequently launched in China in 2020.
Currently, the QCS official website lists over 102 cryborol impurities (please scan the QR code at the end of this article to access the complete list). Our center has conducted comprehensive research on several key impurities of Cryborol, referencing the import registration standard for Cryborol ointment (standard number: JX20200084). The specific structural information regarding critical customer impurities is illustrated in Figure 1.
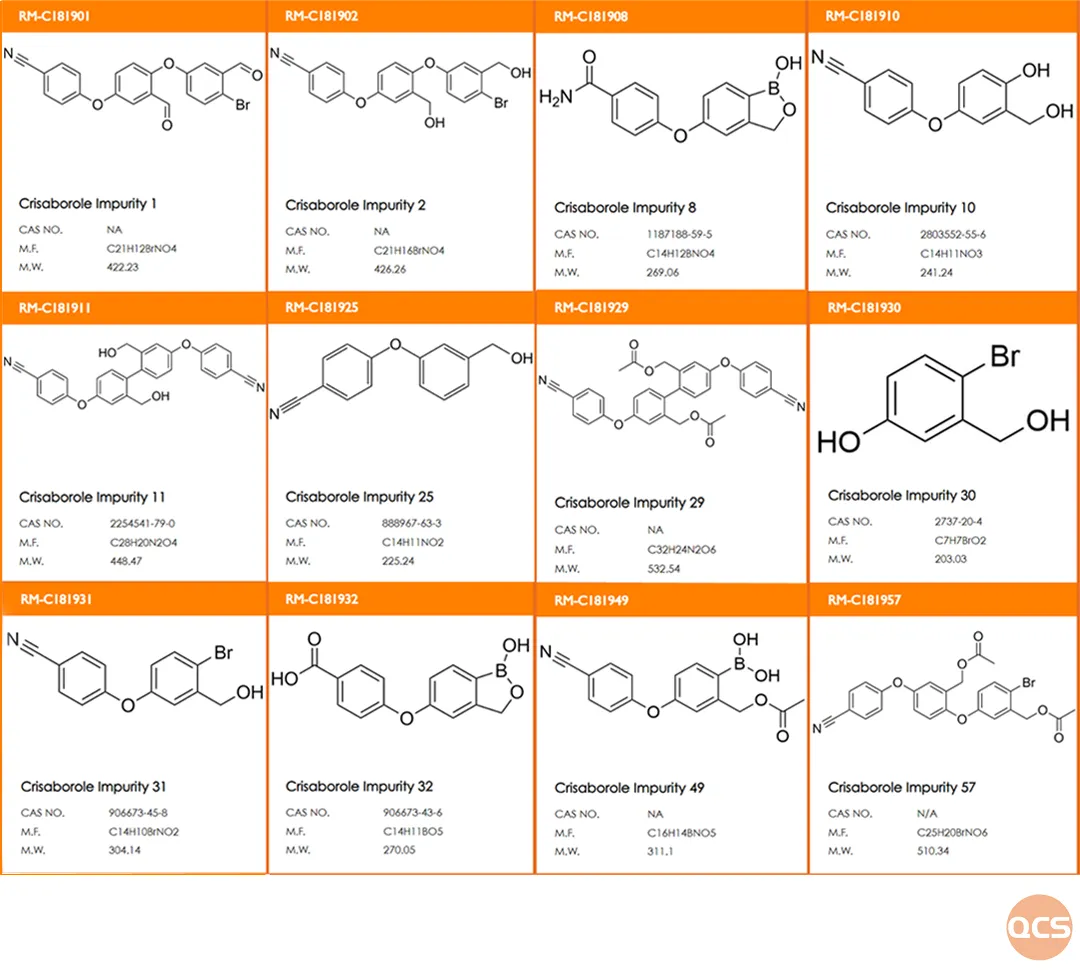
Figure 1: list of impurities relevant to clariborol customers
In accordance with the pertinent substance methodologies outlined in the import registration standard for Creborol ointment (Standard No.: JX20200084), our center primarily conducted qualitative research on the following eleven impurities. The detailed chromatographic data are presented in Figure 2.
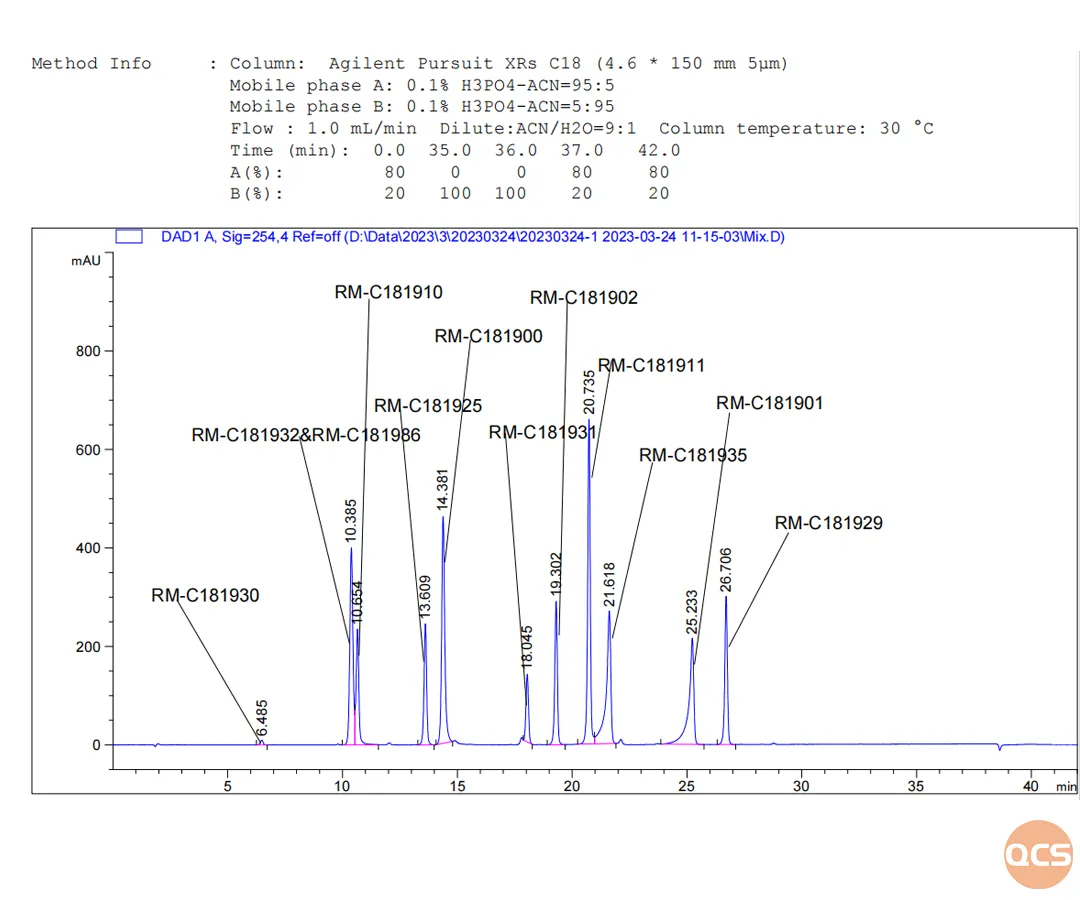
Figure 2: location data of major impurities
As illustrated in Figure 2, impurities 32 and 86 exhibit significant overlap and cannot be effectively separated. This phenomenon is hypothesized to arise from structural similarities between the two compounds, coupled with a minimal overall polarity difference. Consequently, the research and development center's experimental team performed separate injections of each sample; the resulting injection data and product structure formulas are presented in Figure 3. The results confirm that under these chromatographic conditions, both impurity 32 and impurity 86 display identical retention times.
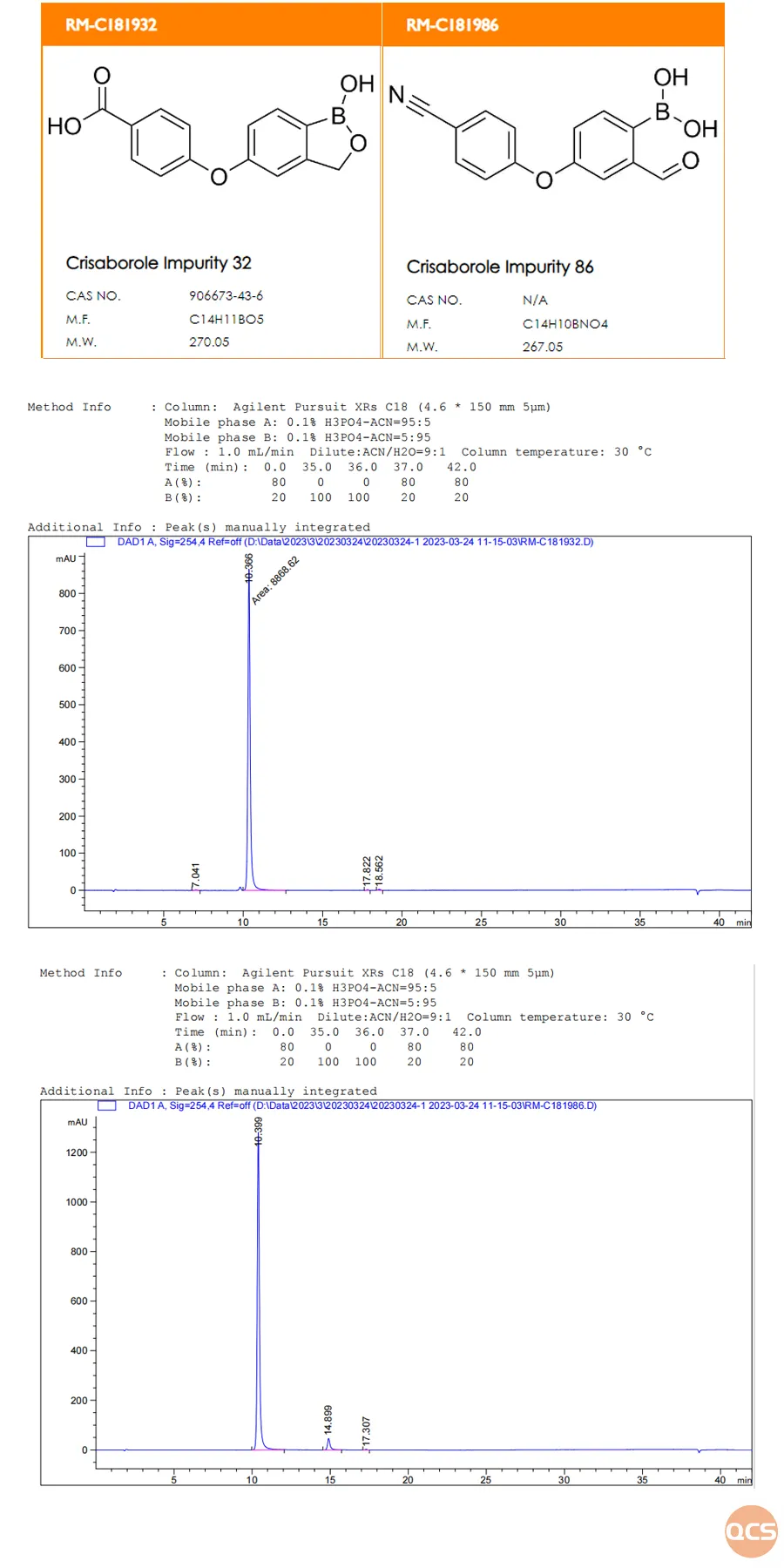
Figure 3: Structural representation and individual injection data for Cleborol impurities 32 and 86
The data indicate that effectively separating cleborol impurity 32 from impurity 86 is challenging under the current chromatographic conditions. This method exhibits limitations in detecting all relevant substances, necessitating optimization to establish a more robust approach for the determination of these substances (We invite customers to engage with our center for further discussions on method optimization).
Interactive Session: In the context of interpreting the relevant substance analysis methods outlined in the Crybolol ointment import registration standard (Standard Number: JX20200084), it has been observed that two coded impurities are specified within this standard: PF-06932644 and PF-06932648. Customers who possess knowledge regarding the structural characteristics of these two codes are encouraged to participate in this activity, which promises exciting surprises!
Q: What are the structural formulas of the two code impurities mentioned in the registration standard: PF-06932644 and PF-06932648?
Please long press the QR code to participate in the prize quiz!



Introduction:
Today, we present a relatively ‘popolar’ drug—Creborol ointment—and its related impurity research. However, data from the Drug Intelligence Network indicates that only the original research manufacturer, Pfizer, has been approved for listing in China, with merely three generic pharmaceutical companies (Hangzhou Industry, Qilu Pharmaceutical, and Nanjing Wanrong Jiancheng & Jiangsu Wanhe) having submitted applications for acceptance. Nevertheless, internal data from Zhenqiang Biological reveal that there are currently over 20 varieties under investigation within research enterprises. This suggests a highly competitive landscape in terms of imitation.
Creborol ointment, a phosphodiesterase 4 inhibitor, is indicated for the topical management of mild to moderate atopic dermatitis in patients aged 2 years and older. In December 2016, Creborol ointment received approval from the US FDA under the trade name Eucrisa and was subsequently launched in China in 2020.
Currently, the QCS official website lists over 102 cryborol impurities (please scan the QR code at the end of this article to access the complete list). Our center has conducted comprehensive research on several key impurities of Cryborol, referencing the import registration standard for Cryborol ointment (standard number: JX20200084). The specific structural information regarding critical customer impurities is illustrated in Figure 1.

Figure 1: list of impurities relevant to clariborol customers
In accordance with the pertinent substance methodologies outlined in the import registration standard for Creborol ointment (Standard No.: JX20200084), our center primarily conducted qualitative research on the following eleven impurities. The detailed chromatographic data are presented in Figure 2.

Figure 2: location data of major impurities
As illustrated in Figure 2, impurities 32 and 86 exhibit significant overlap and cannot be effectively separated. This phenomenon is hypothesized to arise from structural similarities between the two compounds, coupled with a minimal overall polarity difference. Consequently, the research and development center's experimental team performed separate injections of each sample; the resulting injection data and product structure formulas are presented in Figure 3. The results confirm that under these chromatographic conditions, both impurity 32 and impurity 86 display identical retention times.

Figure 3: Structural representation and individual injection data for Cleborol impurities 32 and 86
The data indicate that effectively separating cleborol impurity 32 from impurity 86 is challenging under the current chromatographic conditions. This method exhibits limitations in detecting all relevant substances, necessitating optimization to establish a more robust approach for the determination of these substances (We invite customers to engage with our center for further discussions on method optimization).
Interactive Session: In the context of interpreting the relevant substance analysis methods outlined in the Crybolol ointment import registration standard (Standard Number: JX20200084), it has been observed that two coded impurities are specified within this standard: PF-06932644 and PF-06932648. Customers who possess knowledge regarding the structural characteristics of these two codes are encouraged to participate in this activity, which promises exciting surprises!
Q: What are the structural formulas of the two code impurities mentioned in the registration standard: PF-06932644 and PF-06932648?

Please long press the QR code to participate in the prize quiz!



Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号