Time:2024-01-12
Introduction
Today, we hope to provide assistance to our clients in the study of peroxide impurities in SGLT-2 inhibitors and Gliflozins compounds through research on Empagliflozin peroxide impurities. The two most dazzling stars in SGLT-2 inhibitors are Empagliflozin and Dapagliflozin. According to incomplete statistics, the sales of Boehringer Ingelheim's Empagliflozin exceeded $8.2 billion in 2022, and AstraZeneca's Dapagliflozin sold nearly $4.4 billion in 2022. Information on the series of SGLT-2 inhibitor compounds is shown in Figure 1:
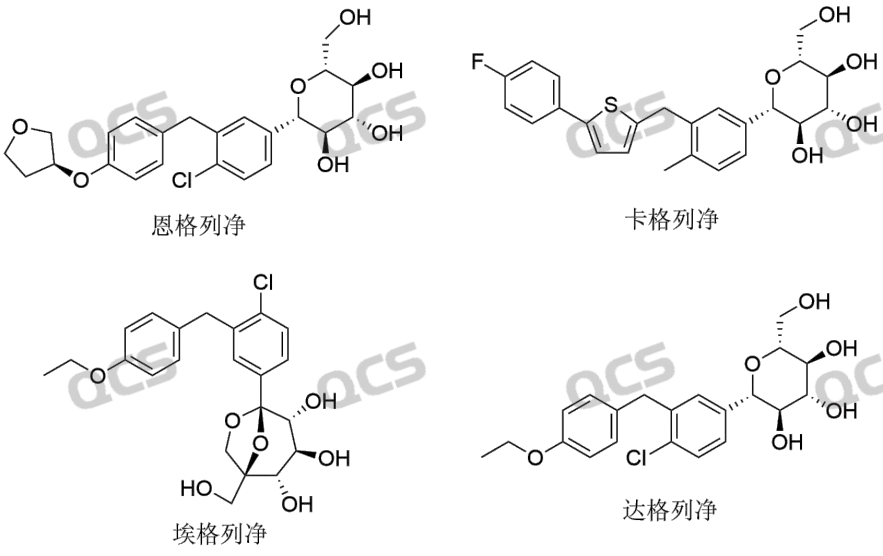
Figure 1: SGLT-2 inhibitor series compound information
Introduction to Empagliflozin hot topics
Currently, Empagliflozin has both single and compound formulations (Empagliflozin metformin), so there are many companies developing and replicating it. According to internal data from ChemStrong, there were over 45 companies that purchased Empagliflozin impurities from our company in 2023 alone. At present, the QCS official website has included a total of 94 impurities of Empagliflozin (scan the QR code at the end of the article to view the list of all impurities). This article will introduce the relevant testing and research work of our center on Empagliflozin peroxide based on the import registration standard (standard number JX20150247) of Empagliflozin tablets.(the structure information of Empagliflozin peroxide is shown in Figure 2:).
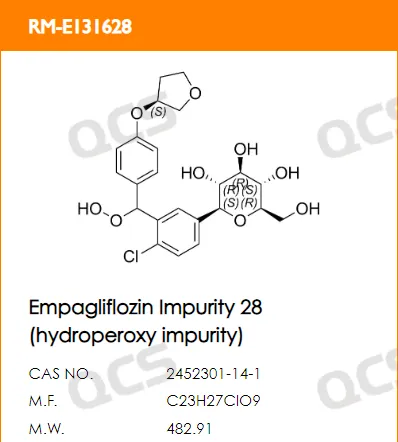
Figure 2: Structural information of Empagliflozin peroxide
Peroxide chromatographic results and structure verification
According to the relevant substance methods in the import registration standard for "Empagliflozin tablets" (standard number JX20150247), our center first conducted a liquid phase qualitative localization study on the peroxide impurity, with chromatographic conditions referring to the relevant substance methods in the standard (see Figure 3)
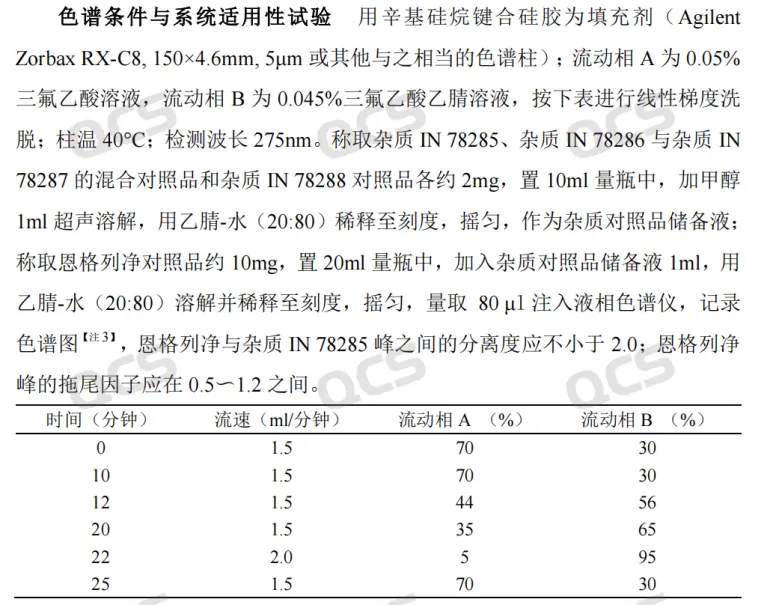
Figure 3: Substance methods related to tablet import registration standards
Referring to this method, our center tested the synthesized Empagliflozin peroxide in the laboratory. The mixed injection results of the peroxide impurity and Empagliflozin raw material are shown in Figure 5, and the injection spectrum of the peroxide impurity alone is shown in Figure 4:
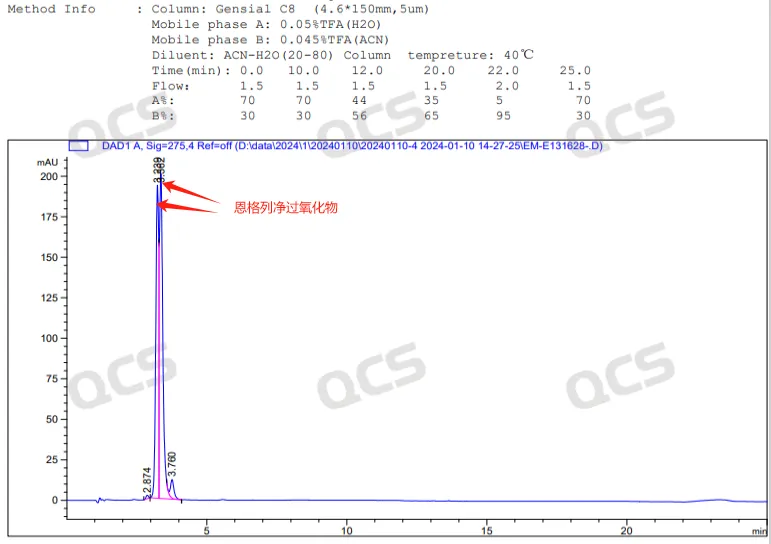
Figure 4: Chromatogram of Empagliflozin peroxide detection
The mixed injection data of Empagliflozin peroxide and Empagliflozin are shown in Figure 5:
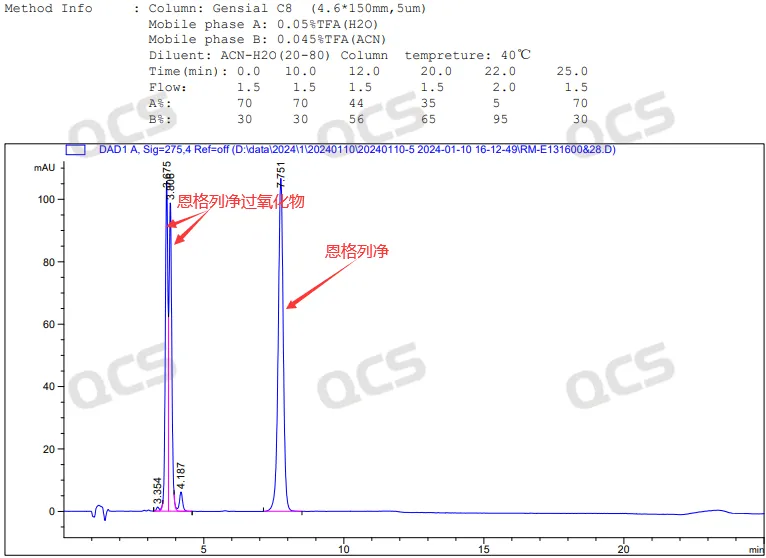
Figure 5: Empagliflozin and its peroxide mixture spectrum
In the above chromatographic results, the retention time of Empagliflozin is 7.7 minutes, and the retention time of peroxide impurities is 3.6-3.8 minutes, showing a bimodal pattern. Due to the fact that peroxide impurities are a mixture of diastereomers (structural formula shown in Figure 6, key chirality marked in red), the imported registration standard conditions have a certain separation effect on this structure, resulting in a bimodal formation.
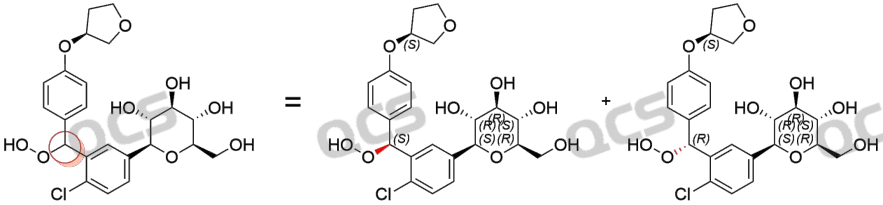
Figure 6: Generate bimodal chiral carbon position map
The QCS R&D center confirmed the sample using liquid chromatography-mass spectrometry (LCMS). Through method optimization, the bimodal results of the impurity sample of Empagliflozin peroxide can be reproduced in LCMS (Figure 7). This conclusion was verified by comparing the MS spectra of the two chromatographic peaks (Figures 8 and 9)
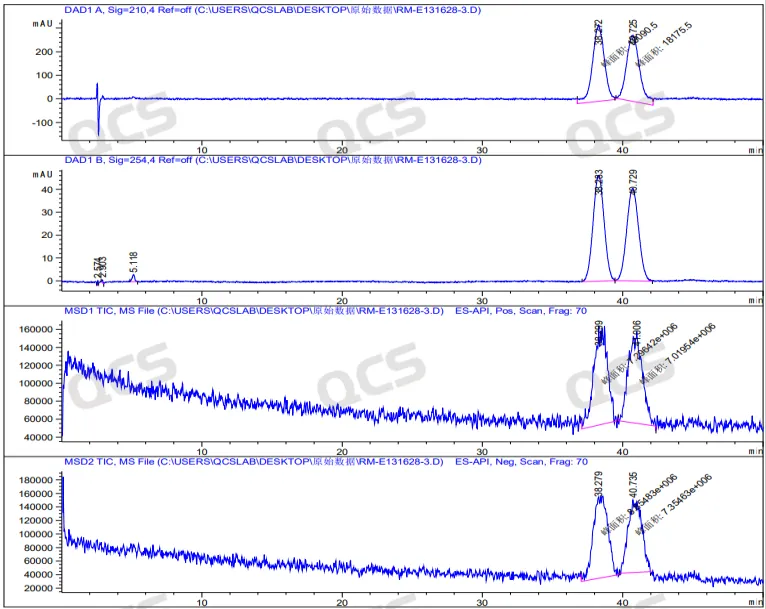
Figure 7: LC-MS spectra of peroxide double peaks
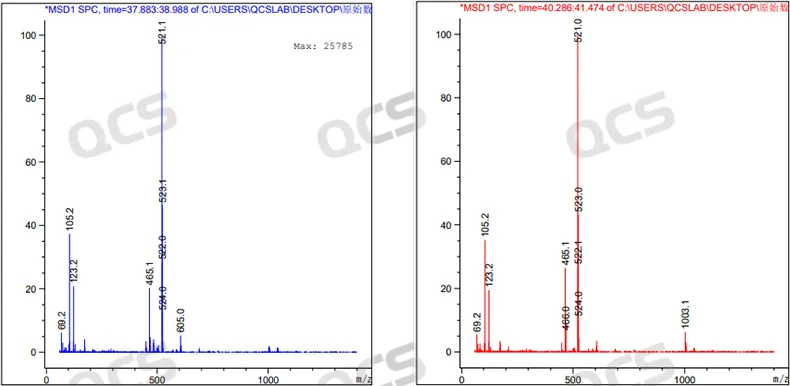
Figure 8: MS results of the chromatographic peaks at RT=38.272 min (left) and RT=40.275 min (right) in the peroxide LC-MS spectrum (mode: Positive ion)
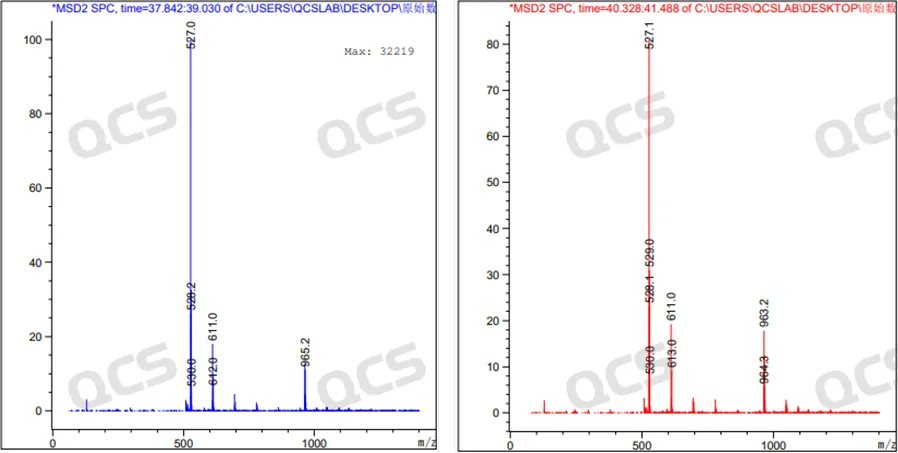
Figure 9: MS results of the chromatographic peaks at RT=38.272 min (left) and RT=40.275 min (right) in the peroxide LC-MS spectrum (mode: negative ion)
From the LC-MS detection data, it can be seen that both chromatographic peaks of Empagliflozin peroxide have a molecular weight of [M+K]+in the positive ion mode and [M+HCOO] - in the negative ion mode. Moreover, in both positive and negative ion modes, the molecular weights of the two chromatographic peaks are the same.
In addition, our research center conducted nuclear magnetic resonance spectroscopy and secondary mass spectrometry testing on the sample, and the nuclear magnetic resonance spectroscopy results of the sample can observe the product structure signal; Clear secondary ion fragmentation structures can be seen in the secondary mass spectrometry results, and the above information is consistent with the product structure. From the nuclear magnetic hydrogen spectrum data, it can also be seen that the product has two sets of peaks, which indirectly proves that the product is a pair of non corresponding isomeric compounds, as shown in Figure 10:
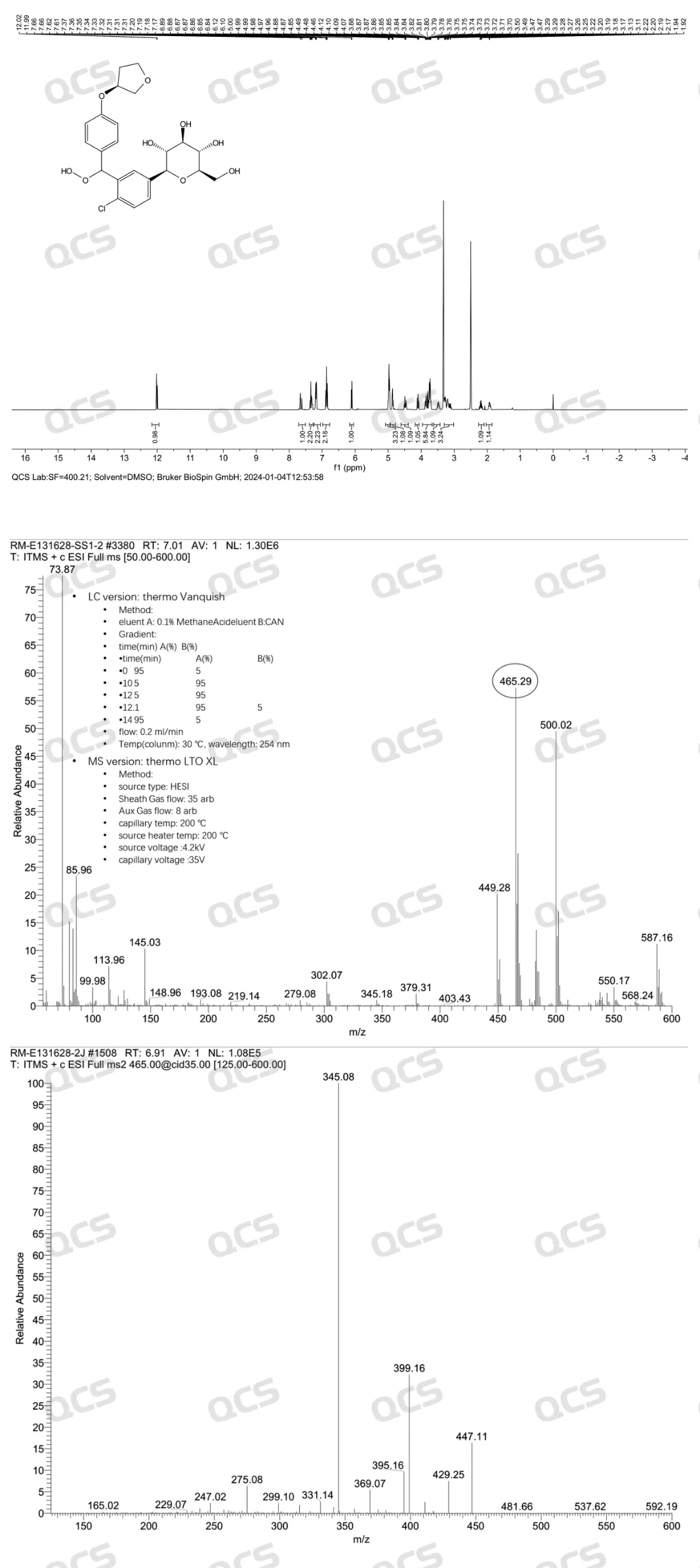
Figure 10: HNMR spectra and secondary mass spectrometry results of Empagliflozin peroxide
Stability test of Empagliflozin peroxide
In addition to the above work, our center also conducted solution stability testing on Empagliflozin peroxide (RM-E131628) according to the relevant substance methods in the import registration standard for Empagliflozin tablets (standard number JX20150247). Samples were injected and tested at 0, 1, 2, 4, 8, 12, and 24 hours after preparation, and the samples were placed at room temperature without avoiding light. The test data is summarized as follows:
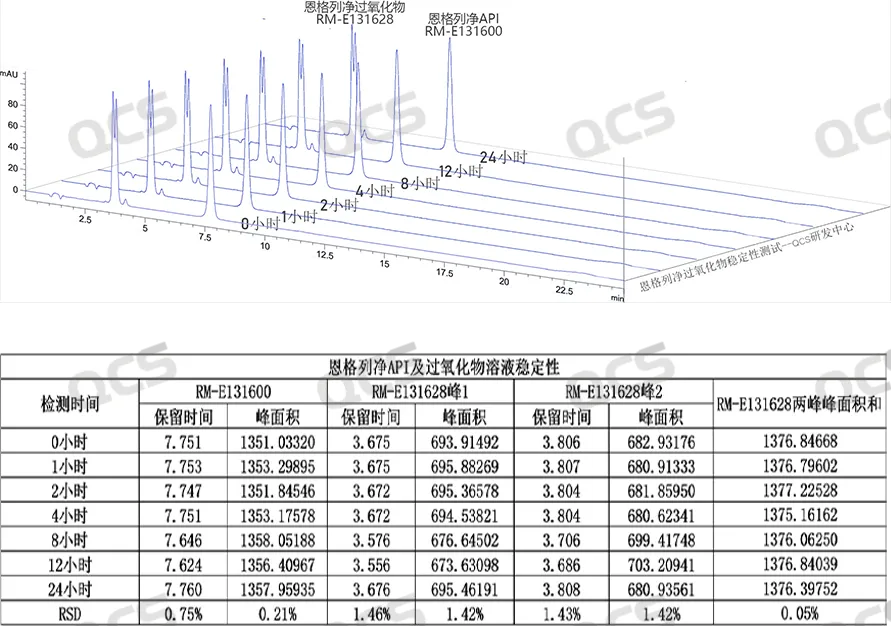
Figure 11: Summary of the stability data of Empagliflozin API (RM-E131600) and Empagliflozin peroxide (RM-E131628) solutions
From Figure 11, it can be seen that the solution of Empagliflozin peroxide (RM-E131628) is relatively stable at room temperature (without avoiding light). It can be seen that the mixed sample of Empagliflozin peroxide and internal standard (Empagliflozin API) was monitored at different time points after sample preparation. The results showed that the impurity had good stability, and there was no significant change in the retention time and peak area of Empagliflozin peroxide peak 1 and peak 2 at different time points (RSD values were all less than 2.0%). This indicates that the sample solution of Empagliflozin peroxide is stable at room temperature, without avoiding light, and under neutral pH conditions.
Additional services and other products
In addition, our center conducted a complete set of nuclear magnetic resonance tests on Empagliflozin peroxide (RM-E131628), including HNMR, CNMR, DEPT, COSY, HSQC, HMBC, and NOESY, and provided relevant spectrum analysis. If the customer intends to purchase our company's Empagliflozin Peroxide (RM-E131628) product, our company will share the CNMR, DEPT, COSY, HSQC, HMBC, and NOESY spectra and the content of spectra analysis with the customer. If you have purchasing intentions, welcome to contact us.
In addition to the impurity products of Empagliflozin peroxide, our center has completed research on Empagliflozin peroxide and Dapagliflozin peroxide. The above products have been validated using relevant registration standard methods. Please contact us if necessary.
Our center has completed the synthesis of four SGLT-2 inhibitors, namely the peroxides of Canagliflozin and Ertugliflozin, which are currently in the stage of collecting detection information. Please feel free to inquire.
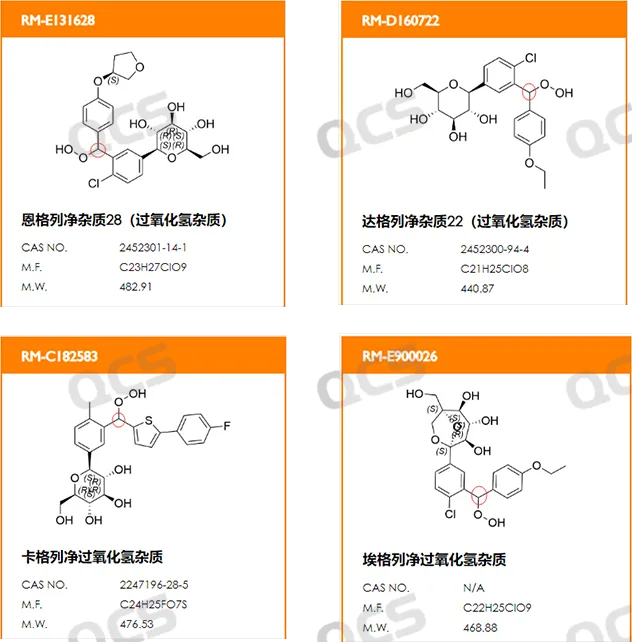
Figure 12: SGLT-2 inhibitor peroxide structural formula information
Empagliflozin peroxide impurity is a very mature product in our center, but we previously received feedback from a customer that there was a conflict with data from other suppliers under MS conditions. Therefore, our center also synchronously tested the MS data and conducted synchronous analysis based on our nuclear magnetic two-dimensional data. Everyone can use it with confidence. Customers who have questions or needs regarding this can contact us.

Introduction
Today, we hope to provide assistance to our clients in the study of peroxide impurities in SGLT-2 inhibitors and Gliflozins compounds through research on Empagliflozin peroxide impurities. The two most dazzling stars in SGLT-2 inhibitors are Empagliflozin and Dapagliflozin. According to incomplete statistics, the sales of Boehringer Ingelheim's Empagliflozin exceeded $8.2 billion in 2022, and AstraZeneca's Dapagliflozin sold nearly $4.4 billion in 2022. Information on the series of SGLT-2 inhibitor compounds is shown in Figure 1:

Figure 1: SGLT-2 inhibitor series compound information
Introduction to Empagliflozin hot topics
Currently, Empagliflozin has both single and compound formulations (Empagliflozin metformin), so there are many companies developing and replicating it. According to internal data from ChemStrong, there were over 45 companies that purchased Empagliflozin impurities from our company in 2023 alone. At present, the QCS official website has included a total of 94 impurities of Empagliflozin (scan the QR code at the end of the article to view the list of all impurities). This article will introduce the relevant testing and research work of our center on Empagliflozin peroxide based on the import registration standard (standard number JX20150247) of Empagliflozin tablets.(the structure information of Empagliflozin peroxide is shown in Figure 2:).

Figure 2: Structural information of Empagliflozin peroxide
Peroxide chromatographic results and structure verification
According to the relevant substance methods in the import registration standard for "Empagliflozin tablets" (standard number JX20150247), our center first conducted a liquid phase qualitative localization study on the peroxide impurity, with chromatographic conditions referring to the relevant substance methods in the standard (see Figure 3)

Figure 3: Substance methods related to tablet import registration standards
Referring to this method, our center tested the synthesized Empagliflozin peroxide in the laboratory. The mixed injection results of the peroxide impurity and Empagliflozin raw material are shown in Figure 5, and the injection spectrum of the peroxide impurity alone is shown in Figure 4:

Figure 4: Chromatogram of Empagliflozin peroxide detection
The mixed injection data of Empagliflozin peroxide and Empagliflozin are shown in Figure 5:

Figure 5: Empagliflozin and its peroxide mixture spectrum
In the above chromatographic results, the retention time of Empagliflozin is 7.7 minutes, and the retention time of peroxide impurities is 3.6-3.8 minutes, showing a bimodal pattern. Due to the fact that peroxide impurities are a mixture of diastereomers (structural formula shown in Figure 6, key chirality marked in red), the imported registration standard conditions have a certain separation effect on this structure, resulting in a bimodal formation.

Figure 6: Generate bimodal chiral carbon position map
The QCS R&D center confirmed the sample using liquid chromatography-mass spectrometry (LCMS). Through method optimization, the bimodal results of the impurity sample of Empagliflozin peroxide can be reproduced in LCMS (Figure 7). This conclusion was verified by comparing the MS spectra of the two chromatographic peaks (Figures 8 and 9)

Figure 7: LC-MS spectra of peroxide double peaks

Figure 8: MS results of the chromatographic peaks at RT=38.272 min (left) and RT=40.275 min (right) in the peroxide LC-MS spectrum (mode: Positive ion)

Figure 9: MS results of the chromatographic peaks at RT=38.272 min (left) and RT=40.275 min (right) in the peroxide LC-MS spectrum (mode: negative ion)
From the LC-MS detection data, it can be seen that both chromatographic peaks of Empagliflozin peroxide have a molecular weight of [M+K]+in the positive ion mode and [M+HCOO] - in the negative ion mode. Moreover, in both positive and negative ion modes, the molecular weights of the two chromatographic peaks are the same.
In addition, our research center conducted nuclear magnetic resonance spectroscopy and secondary mass spectrometry testing on the sample, and the nuclear magnetic resonance spectroscopy results of the sample can observe the product structure signal; Clear secondary ion fragmentation structures can be seen in the secondary mass spectrometry results, and the above information is consistent with the product structure. From the nuclear magnetic hydrogen spectrum data, it can also be seen that the product has two sets of peaks, which indirectly proves that the product is a pair of non corresponding isomeric compounds, as shown in Figure 10:

Figure 10: HNMR spectra and secondary mass spectrometry results of Empagliflozin peroxide
Stability test of Empagliflozin peroxide
In addition to the above work, our center also conducted solution stability testing on Empagliflozin peroxide (RM-E131628) according to the relevant substance methods in the import registration standard for Empagliflozin tablets (standard number JX20150247). Samples were injected and tested at 0, 1, 2, 4, 8, 12, and 24 hours after preparation, and the samples were placed at room temperature without avoiding light. The test data is summarized as follows:

Figure 11: Summary of the stability data of Empagliflozin API (RM-E131600) and Empagliflozin peroxide (RM-E131628) solutions
From Figure 11, it can be seen that the solution of Empagliflozin peroxide (RM-E131628) is relatively stable at room temperature (without avoiding light). It can be seen that the mixed sample of Empagliflozin peroxide and internal standard (Empagliflozin API) was monitored at different time points after sample preparation. The results showed that the impurity had good stability, and there was no significant change in the retention time and peak area of Empagliflozin peroxide peak 1 and peak 2 at different time points (RSD values were all less than 2.0%). This indicates that the sample solution of Empagliflozin peroxide is stable at room temperature, without avoiding light, and under neutral pH conditions.
Additional services and other products
In addition, our center conducted a complete set of nuclear magnetic resonance tests on Empagliflozin peroxide (RM-E131628), including HNMR, CNMR, DEPT, COSY, HSQC, HMBC, and NOESY, and provided relevant spectrum analysis. If the customer intends to purchase our company's Empagliflozin Peroxide (RM-E131628) product, our company will share the CNMR, DEPT, COSY, HSQC, HMBC, and NOESY spectra and the content of spectra analysis with the customer. If you have purchasing intentions, welcome to contact us.
In addition to the impurity products of Empagliflozin peroxide, our center has completed research on Empagliflozin peroxide and Dapagliflozin peroxide. The above products have been validated using relevant registration standard methods. Please contact us if necessary.
Our center has completed the synthesis of four SGLT-2 inhibitors, namely the peroxides of Canagliflozin and Ertugliflozin, which are currently in the stage of collecting detection information. Please feel free to inquire.

Figure 12: SGLT-2 inhibitor peroxide structural formula information
Empagliflozin peroxide impurity is a very mature product in our center, but we previously received feedback from a customer that there was a conflict with data from other suppliers under MS conditions. Therefore, our center also synchronously tested the MS data and conducted synchronous analysis based on our nuclear magnetic two-dimensional data. Everyone can use it with confidence. Customers who have questions or needs regarding this can contact us.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号