Time:2024-10-14
Introduction
Today, we present a stability study on the specific impurities of the atypical antipsychotic drug Epiprazole (briprazole). Epiprazole (briprazole) is classified as an atypical antipsychotic that modulates the monoaminergic neurotransmitter system in the brain. It acts as a partial agonist at serotonin receptor 5-HT1A and dopamine receptors D2 and D3, while also functioning as a partial antagonist at serotonin receptors 5-HT2A and 5-HT2B, as well as adrenergic receptors alpha-1 and alpha-2. Brexpiprazole is a second-generation oral antipsychotic administered once daily, initially discovered by Otsuka and subsequently developed in collaboration with Reibuki. In the United States market, brexpiprazole (brand name: Rexulti) received approval in July 2015 for treating major depressive disorder (MDD) and schizophrenia; it was further approved in 2016 for maintenance treatment of adult patients with schizophrenia. In Europe, brexpiprazole (brand name: Rxulti) gained approval at the end of July 2018.
Experimental Scheme
In this study, our center conducted an investigation into the solution stability of three specific impurities of Epiprazole(bripiprazole), utilizing the chromatographic conditions established by our analytical laboratory. The sample numbers and structural formulas employed are as follows:
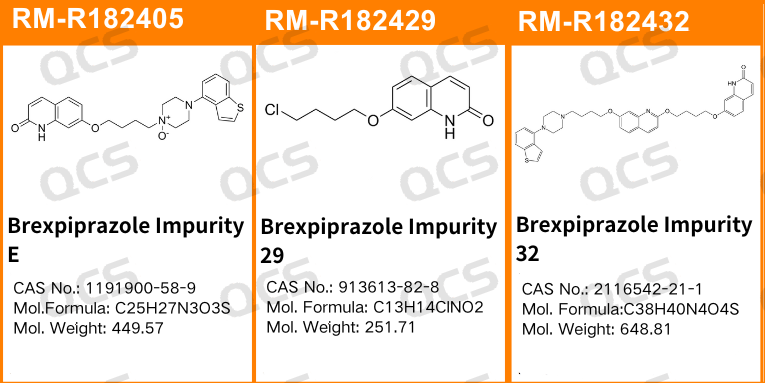
Figure 1: Impurity item numbers and structural formulas utilized in this study
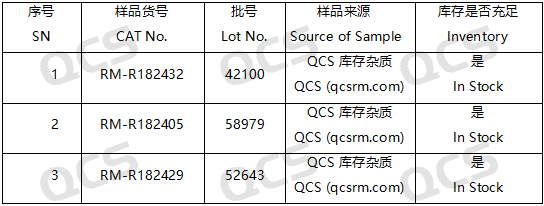
Figure 2: Impurity item numbers and inventory details
In this experiment, our researchers utilized Impurity 32 (Brexpiprazole Impurity 32; CAS NO: 2116542-21-1), RM-R182405 (Brexpiprazole Impurity E; CAS NO: 1191900-58-9), and RM-R182429 (Brexpiprazole Impurity 29; CAS NO: 913613-82-8). An appropriate amount of each impurity was taken and placed in acidic, neutral, and alkaline solutions at room temperature and pressure for durations of 0, 3, 6, 12, and 24 hours. Subsequently, the chromatographic sampling conditions employed in our laboratory's self-developed method were evaluated. The peak area ratios of the main peaks in each spectrum were calculated using the area normalization method. Changes in the ratio of the main peak areas within the chromatograms were observed as a function of storage time for the sample solutions. Based on these observations, we determined the solution stability of each sample. The chromatographic conditions applied in our analysis laboratory's self-developed method are illustrated in Figure 3.
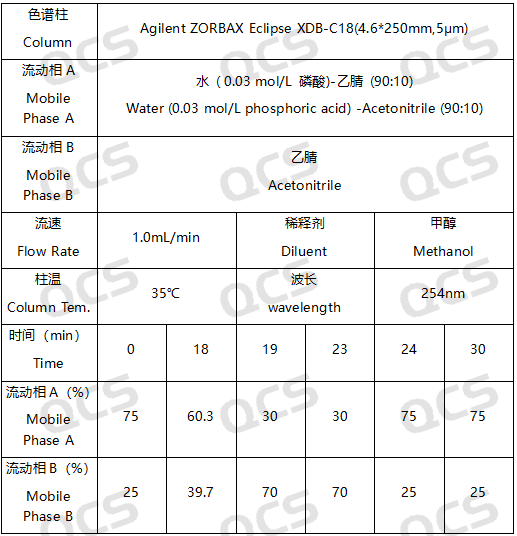
Figure 3: Chromatographic parameters employed for the stability assessment in this experiment
Experimental Conclusion
Following rigorous testing, we determined that the peak-to-peak area ratios of samples RM-R182432, RM-R182405, and RM-R182429 exhibited negligible variation over a 24-hour duration when subjected to acidic, neutral, and alkaline environments. The relative standard deviation was consistently below 2.0%. Thus, it can be concluded that these three samples exhibit significant stability under these conditions for the specified period. The peak area ratios of samples RM-R182432, RM-R182405, and RM-R182429 across various pH levels at each detection point are presented as follows:
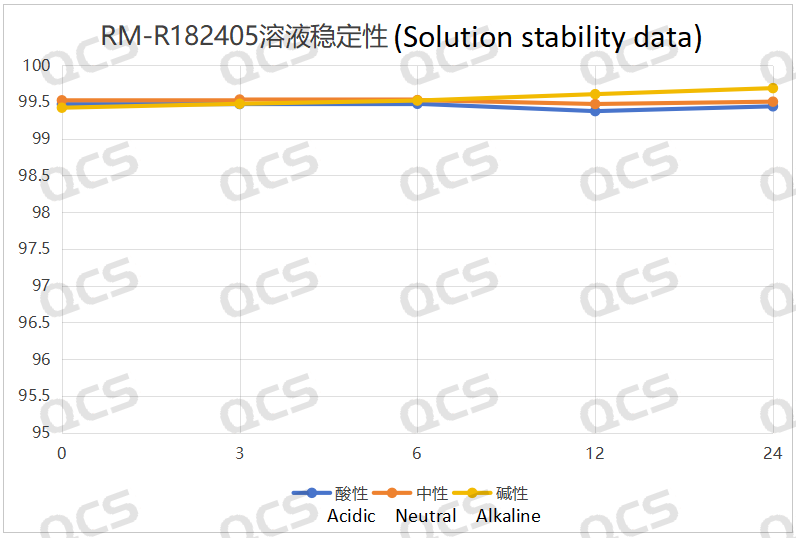
Figure 4: Solution stability data of sample RM-R182405
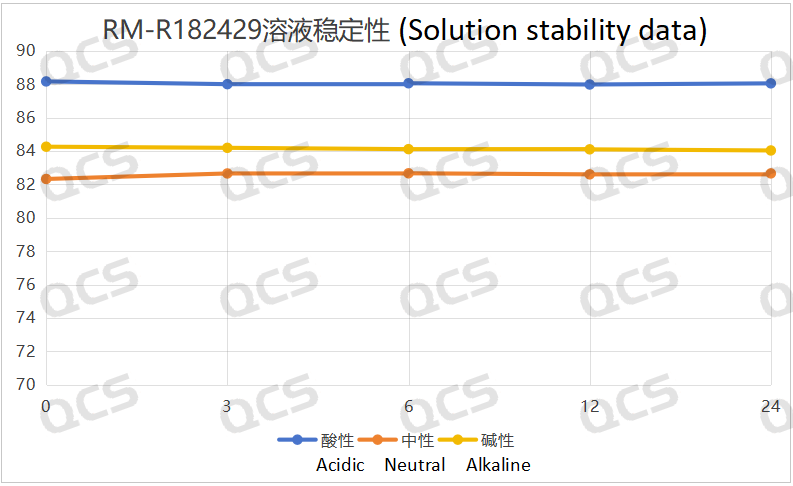
Figure 5: Solution stability data of sample RM-R182429
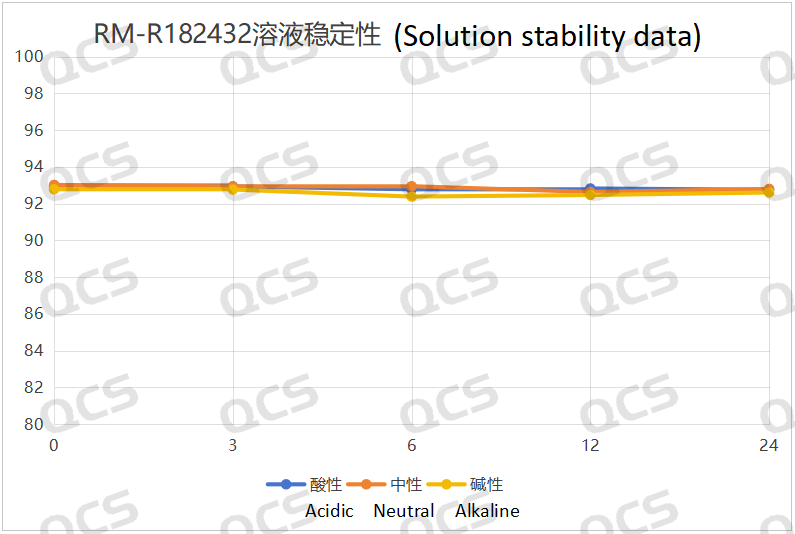
Figure 6: Solution stability data of sample RM-R182432
Summary
In summary, our experiment's findings show that samples RM-R182432, RM-R182405, and RM-R182429 exhibit strong stability in acidic, alkaline, and neutral environments. If you would like more detailed information about the stability of these three samples, please feel free to reach out to the company.


Introduction
Today, we present a stability study on the specific impurities of the atypical antipsychotic drug Epiprazole (briprazole). Epiprazole (briprazole) is classified as an atypical antipsychotic that modulates the monoaminergic neurotransmitter system in the brain. It acts as a partial agonist at serotonin receptor 5-HT1A and dopamine receptors D2 and D3, while also functioning as a partial antagonist at serotonin receptors 5-HT2A and 5-HT2B, as well as adrenergic receptors alpha-1 and alpha-2. Brexpiprazole is a second-generation oral antipsychotic administered once daily, initially discovered by Otsuka and subsequently developed in collaboration with Reibuki. In the United States market, brexpiprazole (brand name: Rexulti) received approval in July 2015 for treating major depressive disorder (MDD) and schizophrenia; it was further approved in 2016 for maintenance treatment of adult patients with schizophrenia. In Europe, brexpiprazole (brand name: Rxulti) gained approval at the end of July 2018.
Experimental Scheme
In this study, our center conducted an investigation into the solution stability of three specific impurities of Epiprazole(bripiprazole), utilizing the chromatographic conditions established by our analytical laboratory. The sample numbers and structural formulas employed are as follows:

Figure 1: Impurity item numbers and structural formulas utilized in this study

Figure 2: Impurity item numbers and inventory details
In this experiment, our researchers utilized Impurity 32 (Brexpiprazole Impurity 32; CAS NO: 2116542-21-1), RM-R182405 (Brexpiprazole Impurity E; CAS NO: 1191900-58-9), and RM-R182429 (Brexpiprazole Impurity 29; CAS NO: 913613-82-8). An appropriate amount of each impurity was taken and placed in acidic, neutral, and alkaline solutions at room temperature and pressure for durations of 0, 3, 6, 12, and 24 hours. Subsequently, the chromatographic sampling conditions employed in our laboratory's self-developed method were evaluated. The peak area ratios of the main peaks in each spectrum were calculated using the area normalization method. Changes in the ratio of the main peak areas within the chromatograms were observed as a function of storage time for the sample solutions. Based on these observations, we determined the solution stability of each sample. The chromatographic conditions applied in our analysis laboratory's self-developed method are illustrated in Figure 3.

Figure 3: Chromatographic parameters employed for the stability assessment in this experiment
Experimental Conclusion
Following rigorous testing, we determined that the peak-to-peak area ratios of samples RM-R182432, RM-R182405, and RM-R182429 exhibited negligible variation over a 24-hour duration when subjected to acidic, neutral, and alkaline environments. The relative standard deviation was consistently below 2.0%. Thus, it can be concluded that these three samples exhibit significant stability under these conditions for the specified period. The peak area ratios of samples RM-R182432, RM-R182405, and RM-R182429 across various pH levels at each detection point are presented as follows:

Figure 4: Solution stability data of sample RM-R182405

Figure 5: Solution stability data of sample RM-R182429

Figure 6: Solution stability data of sample RM-R182432
Summary
In summary, our experiment's findings show that samples RM-R182432, RM-R182405, and RM-R182429 exhibit strong stability in acidic, alkaline, and neutral environments. If you would like more detailed information about the stability of these three samples, please feel free to reach out to the company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号