Time:2024-10-09
Introduction
Today, we present a study on the stability of specific impurities in Dapoxetine, a pharmacological treatment for premature ejaculation (PE) in men aged 18 to 64 years who experience significant personal distress, interpersonal challenges, and inadequate ejaculatory control due to PE.
Experimental Scheme
In this study, our center conducted an investigation into the solution stability of three specific impurities in Dapoxetine hydrochloride, referencing the chromatographic conditions outlined under the “Related Substances” check item in the import registration standard for Dapoxetine Hydrochloride Tablets (Standard Number: JX20150184). The sample numbers and structural formulas utilized are presented in FIG. 1 and FIG. 2.
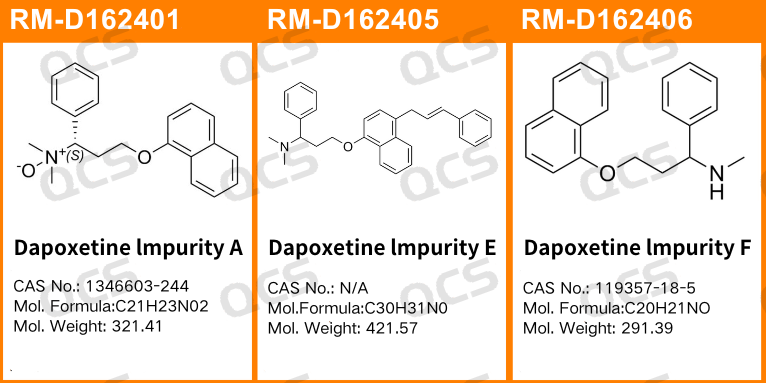
Figure 1: The number and structure of impurity items utilized in this study
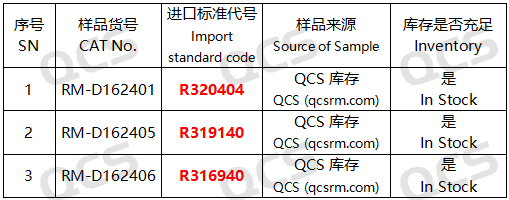
Figure 2: The correlation between the impurity codes specified in the standard and the impurity item numbers employed in this research
In this study, the research and development personnel took appropriate amounts of RM-D162401 (R320404; Dapoxetine Impurity A; CAS NO: 1346603-24-4), RM-D162405 (R319140; Dapoxetine Impurity E), and RM-D162406 (R316940; Dapoxetine Impurity F; CAS NO: 119357-18-5) and placed them in acidic, neutral, and alkaline solutions at room temperature and atmospheric pressure for durations of 0, 3, 6, 12, and 24 hours. This procedure followed the chromatographic conditions outlined under the check item "Related substances" in the import registration standard for Dapoxetine Hydrochloride Tablets (Standard No. JX20150184). The stability of the sample solution will be assessed by monitoring changes in the main peak area on the chromatogram as a function of storage time.
Experimental Results
Upon testing, we observed that the peak-to-peak area of samples RM-D162401 (R320404), RM-D162405 (R319140), and RM-D162406 (R316940) exhibited minimal variation over a 24-hour period in acidic, neutral, and alkaline solutions, with a relative standard deviation of less than 2.0%. Consequently, these three samples can be regarded as relatively stable when subjected to acidic, neutral, and alkaline environments for 24 hours. The main peak area data for each detection point of the aforementioned samples across different pH values is presented below:
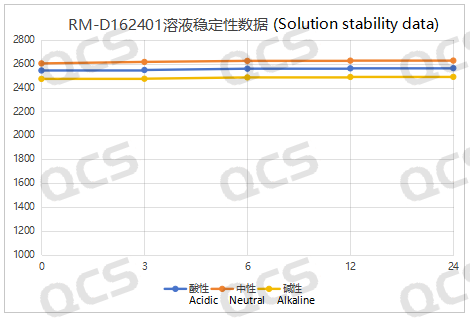
Figure 3: Schematic representation of the stability data summary for sample RM-D162401
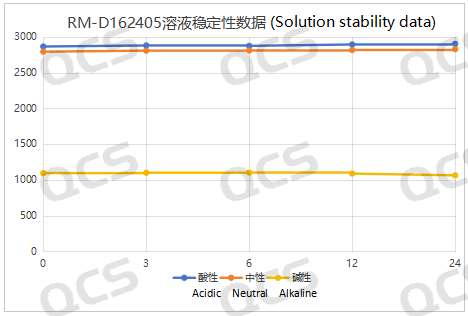
Figure 4: Schematic representation of the summary data on solution stability for sample RM-D162405
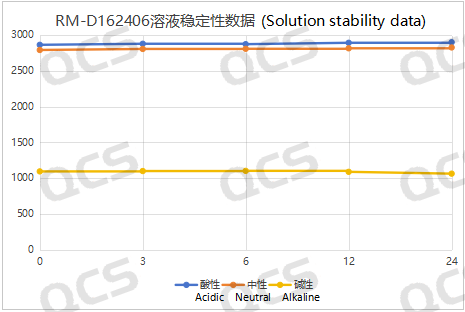
Figure 5: Schematic representation of the stability data summary for sample RM-D162406
Experimental conclusion
In conclusion, our experiment demonstrated that the three specific impurity samples of Dapoxetine—RM-D162401 (R320404), RM-D162405 (R319140), and RM-D162406 (R316940)—exhibited commendable stability across acidic, alkaline, and neutral solutions. For inquiries concerning the stability characteristics of these three samples, please do not hesitate to contact our company.


Introduction
Today, we present a study on the stability of specific impurities in Dapoxetine, a pharmacological treatment for premature ejaculation (PE) in men aged 18 to 64 years who experience significant personal distress, interpersonal challenges, and inadequate ejaculatory control due to PE.
Experimental Scheme
In this study, our center conducted an investigation into the solution stability of three specific impurities in Dapoxetine hydrochloride, referencing the chromatographic conditions outlined under the “Related Substances” check item in the import registration standard for Dapoxetine Hydrochloride Tablets (Standard Number: JX20150184). The sample numbers and structural formulas utilized are presented in FIG. 1 and FIG. 2.

Figure 1: The number and structure of impurity items utilized in this study

Figure 2: The correlation between the impurity codes specified in the standard and the impurity item numbers employed in this research
In this study, the research and development personnel took appropriate amounts of RM-D162401 (R320404; Dapoxetine Impurity A; CAS NO: 1346603-24-4), RM-D162405 (R319140; Dapoxetine Impurity E), and RM-D162406 (R316940; Dapoxetine Impurity F; CAS NO: 119357-18-5) and placed them in acidic, neutral, and alkaline solutions at room temperature and atmospheric pressure for durations of 0, 3, 6, 12, and 24 hours. This procedure followed the chromatographic conditions outlined under the check item "Related substances" in the import registration standard for Dapoxetine Hydrochloride Tablets (Standard No. JX20150184). The stability of the sample solution will be assessed by monitoring changes in the main peak area on the chromatogram as a function of storage time.
Experimental Results
Upon testing, we observed that the peak-to-peak area of samples RM-D162401 (R320404), RM-D162405 (R319140), and RM-D162406 (R316940) exhibited minimal variation over a 24-hour period in acidic, neutral, and alkaline solutions, with a relative standard deviation of less than 2.0%. Consequently, these three samples can be regarded as relatively stable when subjected to acidic, neutral, and alkaline environments for 24 hours. The main peak area data for each detection point of the aforementioned samples across different pH values is presented below:

Figure 3: Schematic representation of the stability data summary for sample RM-D162401

Figure 4: Schematic representation of the summary data on solution stability for sample RM-D162405

Figure 5: Schematic representation of the stability data summary for sample RM-D162406
Experimental conclusion
In conclusion, our experiment demonstrated that the three specific impurity samples of Dapoxetine—RM-D162401 (R320404), RM-D162405 (R319140), and RM-D162406 (R316940)—exhibited commendable stability across acidic, alkaline, and neutral solutions. For inquiries concerning the stability characteristics of these three samples, please do not hesitate to contact our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号