Time:2024-09-27


Introduction
Isavuconazonium sulfate is a novel therapeutic agent for the management of invasive aspergillosis and invasive mucormycosis in adult patients. This drug was collaboratively developed by Basilea Pharmaceutical Co., Ltd. from Switzerland and Astellas Pharma Inc. from Japan, receiving approval from the U.S. Food and Drug Administration (FDA) on March 6, 2015. Additionally, Isavuconazole sulfate serves as a prodrug for the triazole antifungal agent Isavuconazole.
Discover of Impurities
Recently, we have received substantial customer feedback during the research phase of Isavuconazole sulfate raw materials and formulations, indicating a significant presence of degradation impurities, primarily attributed to high-temperature conditions. According to this feedback, impurity levels may exceed 0.5% under elevated temperature experimental settings. Customers are interested in collaborating with us to synthesize and characterize these impurities effectively in order to manage degradation more efficiently. Below is a summary of test data and impurity characteristics provided by several customers (Figure 1):
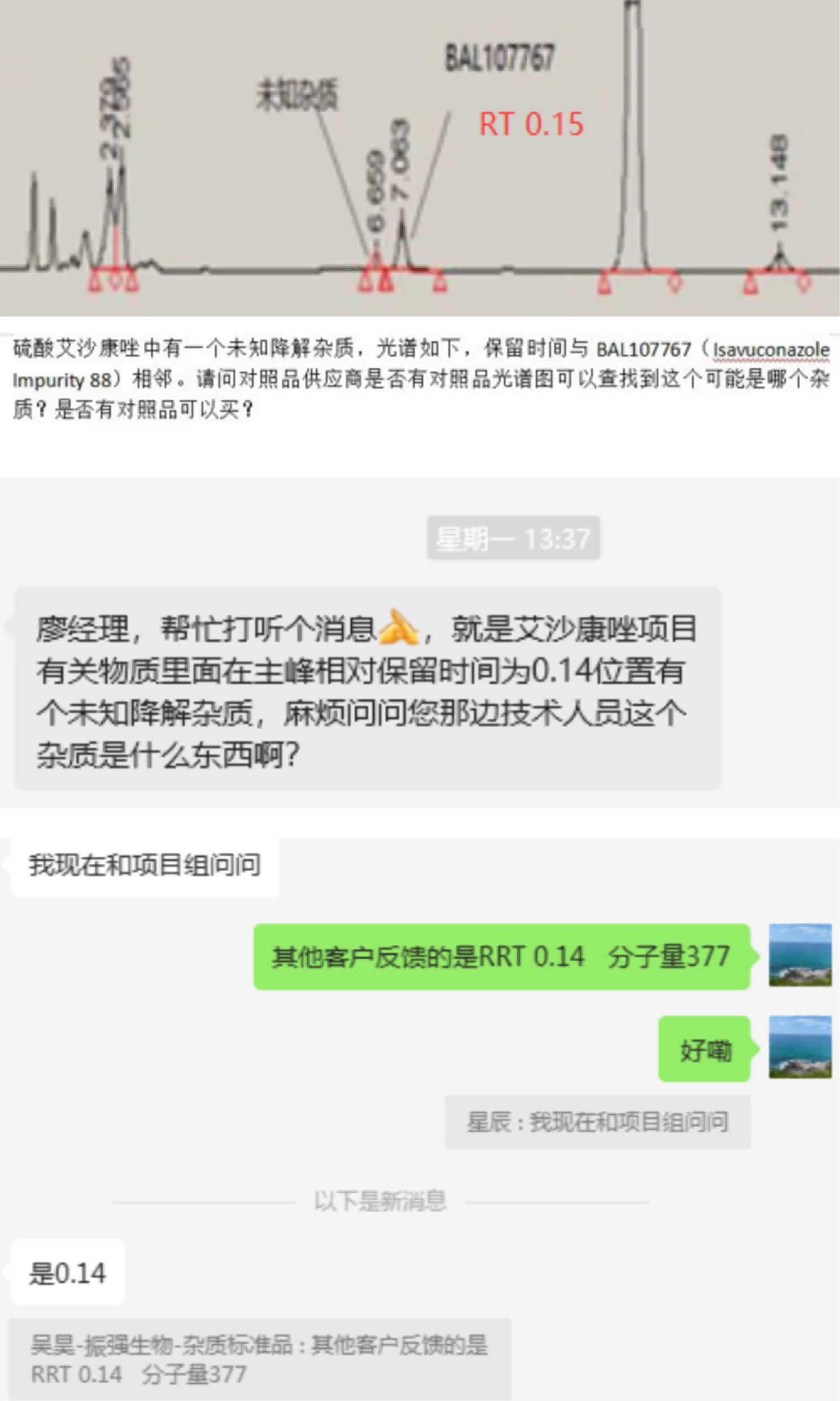
Figure 1: summary of thermal degradation impurity information with feedback from multiple customers
The Production of Impurities
The QCS Standard Material R&D Center initially received a customer inquiry regarding the thermal degradation impurity of Isavuconazole sulfate at the end of 2023, during which time the specific structure of the impurity was still unknown and remained in an exploratory phase.
Based on customer feedback and the information we have collected through various channels, this impurity has the following characteristics:

1. The impurity peaks observed in front of BAL107767 exhibit a relative retention time (RRT) of approximately 0.14.
2. In the negative ion mode of mass spectrometry, an impurity manifests as a signal at m/z 376.
3. Both raw materials and formulations are susceptible to degradation.
4. The concentration of impurities significantly increases under elevated temperature and alkaline conditions.
5. Impurities demonstrate poor stability, posing challenges for transportation and storage conditions.
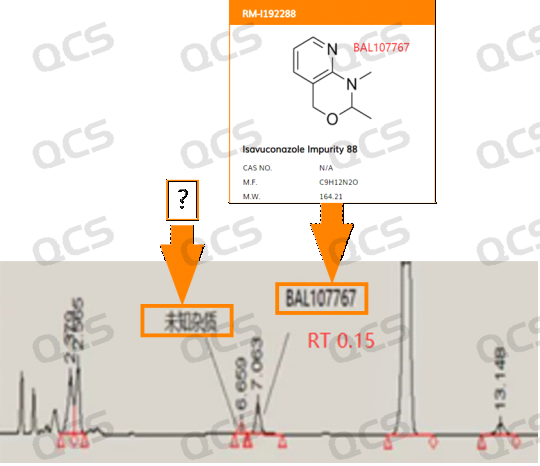
Figure 2: Isavuconazole sulfate thermal degradation impurities and code impurity BAL107767 liquid chromatography reference
With the attention of many research and development institutions on the impurity, the research on the impurity has been deepened, and the accurate chemical structure of the degraded impurity has been inferred by various means. In order to meet the needs of our customers for the verification of the impurity structure, our center has started the full synthesis of the impurity from around March this year based on the speculated chemical structure. After 5 months of synthetic exploration and continuous failures and summaries, the QCS Standard Material Research and Development Center finally completed the production of the degradation impurity recently.

Figure 3: thermal degradation impurity sample of QCS central Isavuconazole sulfate
Research on Impurities
After nearly five months of synthetic exploration and experimentation, the QCS Standard Material Research and Development Center successfully synthesized high-purity Isavuconazole sulfate thermal degradation impurity samples. The standard for this directional synthesis exhibits exceptional purity and favorable physicochemical properties. This impurity standard appears as a white to off-white powder solid that demonstrates excellent flowability under sealed conditions (FIG. 3, left).
Simultaneously, the known code impurity BAL107767 was utilized as the localization peak in our laboratory. Under defined chromatographic conditions, the retention time of the thermal degradation impurity standard generated by our center aligned with that of the impurity peak observed in both API and preparation samples (FIG. 6). The quality spectrum results for the impurity standard were consistent with those of the impurity peak found in API/preparation samples (FIG. 4). The sample underwent analysis using nuclear magnetic resonance spectroscopy, liquid chromatography, mass spectrometry, and ultraviolet absorption spectroscopy.
The following are some data screenshots pertaining to thermal degradation impurity standards produced by QCS Standard Material R&D Center (FIG. 4-FIG. 6):

Figure 4: Mass spectrometry data illustrating the thermal degradation of impurities (anion model).
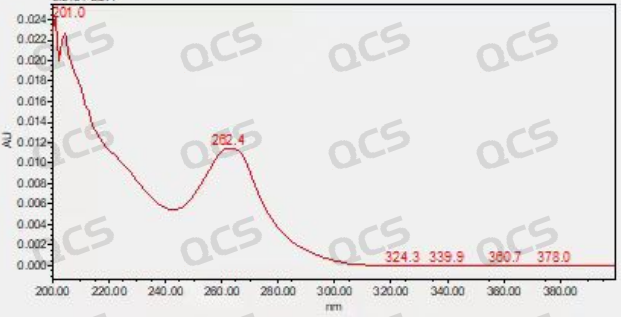
Figure 5: ultraviolet absorption spectra depicting the thermal degradation of impurities
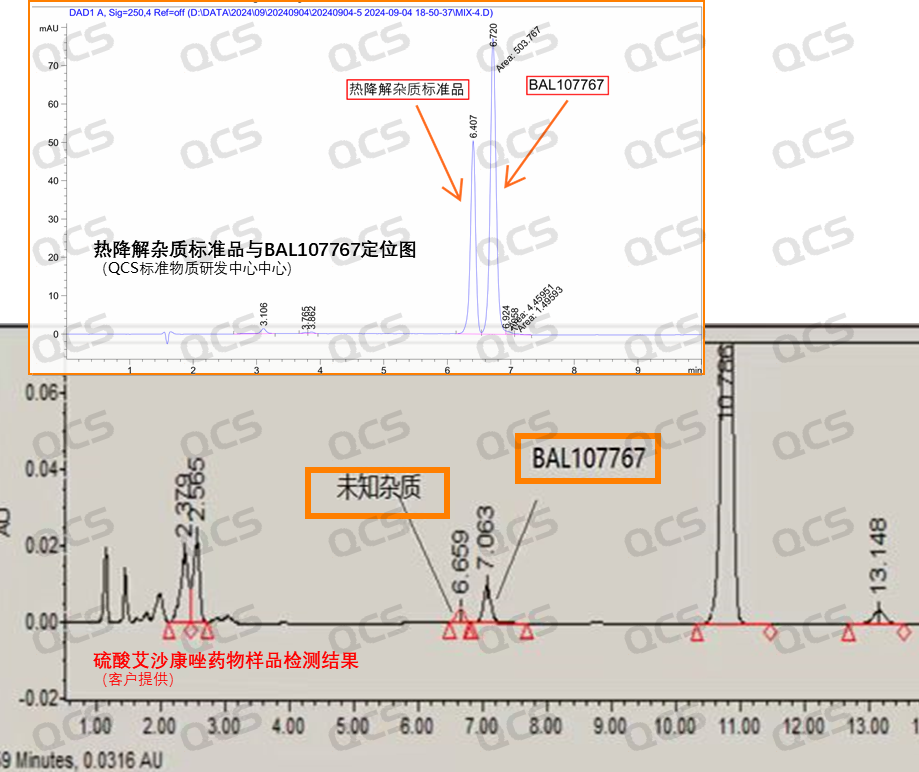
Figure 6: liquid phase validation data for thermal degradation impurity standards
Notice: Our recent research into the location of this impurity has revealed why some customers did not observe its presence. Specifically, when the import registration method is meticulously adhered to, there is a significant likelihood that the degraded impurity will overlap with BAL107767. The chromatogram presented in Figure 6 illustrates that our center has optimized the methodology based on the import registration standards for Isavuconazole sulfate for injection. Consequently, effective separation between degradation impurities and BAL107767 was achieved. Therefore, it is plausible that some customers reported an absence of this impurity due to their focus on changes in BAL107767 and whether the material remains stable. It is essential for analysts to comprehend this without further elaboration.
We are pleased to announce that our center has successfully completed the research on thermal degradation impurities of Isavuconazole, and these identified impurities can facilitate the development of related products. We invite interested customers to reach out for consultations and discussions.

Dr. Wei Xu obtained both his master's degree and doctoral degree from Lanzhou University. His research focuses on the total synthesis of natural products with pharmaceutical activity, as well as methodologies in organic synthesis. He has successfully completed the total synthesis of several complex natural products and has published numerous high-impact SCI papers.
Since joining the QCS Standard Substances R&D Center in 2023, Dr. Wei Xu has taken on the role of leader for the Second Department of Organic Synthesis, where he has focused on researching and developing directed synthesis methodologies for several complex drug impurities. As of March this year, Dr. Wei Xu successfully completed the production of a standard for Isavuconazole sulfate thermal degradation impurities through iterative synthesis attempts and process optimization.




Introduction
Isavuconazonium sulfate is a novel therapeutic agent for the management of invasive aspergillosis and invasive mucormycosis in adult patients. This drug was collaboratively developed by Basilea Pharmaceutical Co., Ltd. from Switzerland and Astellas Pharma Inc. from Japan, receiving approval from the U.S. Food and Drug Administration (FDA) on March 6, 2015. Additionally, Isavuconazole sulfate serves as a prodrug for the triazole antifungal agent Isavuconazole.
Discover of Impurities
Recently, we have received substantial customer feedback during the research phase of Isavuconazole sulfate raw materials and formulations, indicating a significant presence of degradation impurities, primarily attributed to high-temperature conditions. According to this feedback, impurity levels may exceed 0.5% under elevated temperature experimental settings. Customers are interested in collaborating with us to synthesize and characterize these impurities effectively in order to manage degradation more efficiently. Below is a summary of test data and impurity characteristics provided by several customers (Figure 1):

Figure 1: summary of thermal degradation impurity information with feedback from multiple customers
The Production of Impurities
The QCS Standard Material R&D Center initially received a customer inquiry regarding the thermal degradation impurity of Isavuconazole sulfate at the end of 2023, during which time the specific structure of the impurity was still unknown and remained in an exploratory phase.
Based on customer feedback and the information we have collected through various channels, this impurity has the following characteristics:

1. The impurity peaks observed in front of BAL107767 exhibit a relative retention time (RRT) of approximately 0.14.
2. In the negative ion mode of mass spectrometry, an impurity manifests as a signal at m/z 376.
3. Both raw materials and formulations are susceptible to degradation.
4. The concentration of impurities significantly increases under elevated temperature and alkaline conditions.
5. Impurities demonstrate poor stability, posing challenges for transportation and storage conditions.

Figure 2: Isavuconazole sulfate thermal degradation impurities and code impurity BAL107767 liquid chromatography reference
With the attention of many research and development institutions on the impurity, the research on the impurity has been deepened, and the accurate chemical structure of the degraded impurity has been inferred by various means. In order to meet the needs of our customers for the verification of the impurity structure, our center has started the full synthesis of the impurity from around March this year based on the speculated chemical structure. After 5 months of synthetic exploration and continuous failures and summaries, the QCS Standard Material Research and Development Center finally completed the production of the degradation impurity recently.

Figure 3: thermal degradation impurity sample of QCS central Isavuconazole sulfate
Research on Impurities
After nearly five months of synthetic exploration and experimentation, the QCS Standard Material Research and Development Center successfully synthesized high-purity Isavuconazole sulfate thermal degradation impurity samples. The standard for this directional synthesis exhibits exceptional purity and favorable physicochemical properties. This impurity standard appears as a white to off-white powder solid that demonstrates excellent flowability under sealed conditions (FIG. 3, left).
Simultaneously, the known code impurity BAL107767 was utilized as the localization peak in our laboratory. Under defined chromatographic conditions, the retention time of the thermal degradation impurity standard generated by our center aligned with that of the impurity peak observed in both API and preparation samples (FIG. 6). The quality spectrum results for the impurity standard were consistent with those of the impurity peak found in API/preparation samples (FIG. 4). The sample underwent analysis using nuclear magnetic resonance spectroscopy, liquid chromatography, mass spectrometry, and ultraviolet absorption spectroscopy.
The following are some data screenshots pertaining to thermal degradation impurity standards produced by QCS Standard Material R&D Center (FIG. 4-FIG. 6):

Figure 4: Mass spectrometry data illustrating the thermal degradation of impurities (anion model).

Figure 5: ultraviolet absorption spectra depicting the thermal degradation of impurities

Figure 6: liquid phase validation data for thermal degradation impurity standards
Notice: Our recent research into the location of this impurity has revealed why some customers did not observe its presence. Specifically, when the import registration method is meticulously adhered to, there is a significant likelihood that the degraded impurity will overlap with BAL107767. The chromatogram presented in Figure 6 illustrates that our center has optimized the methodology based on the import registration standards for Isavuconazole sulfate for injection. Consequently, effective separation between degradation impurities and BAL107767 was achieved. Therefore, it is plausible that some customers reported an absence of this impurity due to their focus on changes in BAL107767 and whether the material remains stable. It is essential for analysts to comprehend this without further elaboration.
We are pleased to announce that our center has successfully completed the research on thermal degradation impurities of Isavuconazole, and these identified impurities can facilitate the development of related products. We invite interested customers to reach out for consultations and discussions.

Dr. Wei Xu obtained both his master's degree and doctoral degree from Lanzhou University. His research focuses on the total synthesis of natural products with pharmaceutical activity, as well as methodologies in organic synthesis. He has successfully completed the total synthesis of several complex natural products and has published numerous high-impact SCI papers.
Since joining the QCS Standard Substances R&D Center in 2023, Dr. Wei Xu has taken on the role of leader for the Second Department of Organic Synthesis, where he has focused on researching and developing directed synthesis methodologies for several complex drug impurities. As of March this year, Dr. Wei Xu successfully completed the production of a standard for Isavuconazole sulfate thermal degradation impurities through iterative synthesis attempts and process optimization.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号