Time:2024-09-20


Today, we will share a study on the stability of Antithrombotic drug-Apixaban specific impurities. Apixaban is clinically used in adult patients undergoing hip or knee replacement surgery to prevent venous thromboembolism (VTE) events.
Experimental plan
In this experiment, Our center conducted a solution stability study on five specific impurities of Apixaban using the chromatographic conditions used under the "Analysis" section of the "Apixaban" variety in the United States Pharmacopeia. The sample item number and structural formula used are shown in Figure 1 and Figure 2 below:
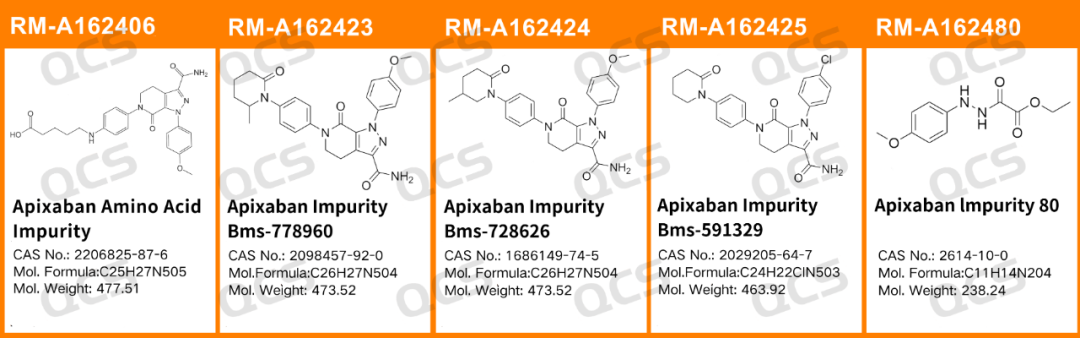
Figure 1: The impurity item number and structure used in this study
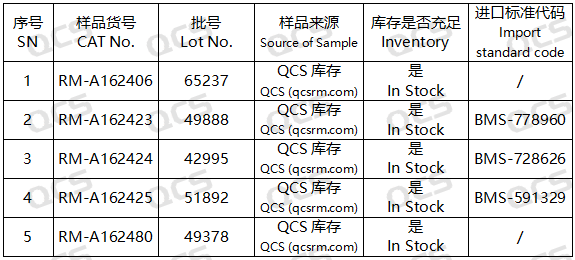
Figure 2: Corresponding relationship diagram between impurity codes included in the standard and impurity item numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-A162406(Apixaban Amino Acid Impurity; CAS# 2206825-87-6), RM-A162423(Apixaban Impurity Bms-778960; CAS#2098457-92-0), RM-A162424(Apixaban Impurity Bms-728626; CAS#1686149-74-5), RM-A162425(Apixaban Impurity Bms-591329; CAS#2029205-64-7) and RM-A162480(Apixaban Impurity 80; CAS#2614-10-0), and placed them in acidic, neutral, and alkaline solutions, respectively. They were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours, and then injected for testing according to the chromatographic conditions used under the "Analysis" item of the "Apixaban" variety in the United States Pharmacopeia standard. Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Experimental result
RM-A162406、RM-A162423、RM-A162424和RM-A162425
After testing, it was found that the main peak area of samples RM-A162406, RM-A162423, RM-A162424, and RM-A162425 did not change significantly during 24 hours of storage in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the above four samples are relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The main peak area data of each detection point for these four samples under different pH conditions are as follows. Among them, RM-A162406 showed a ghost peak in the detection data after being placed in neutral solution for 3 hours, which was removed:
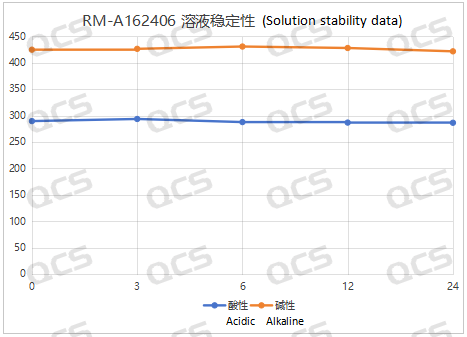
Figure 3: Summary line chart of solution stability data of sample RM-A162406 after being placed in acidic and alkaline solutions for 24 hours
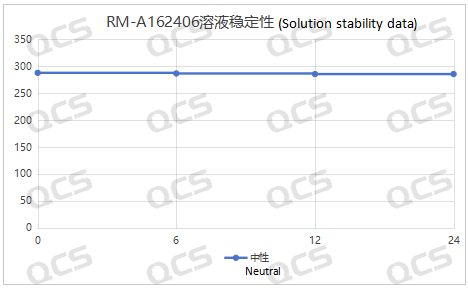
Figure 4: Summary line chart of solution stability data of sample RM-A162406 after being placed in a medium solution for 24 hours
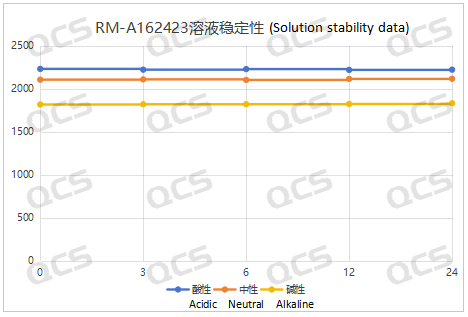
Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-A162423
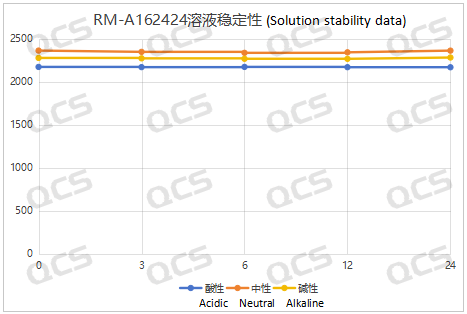
Figure 6: Summary Line Chart of Solution Stability Data for Sample RM-A162424
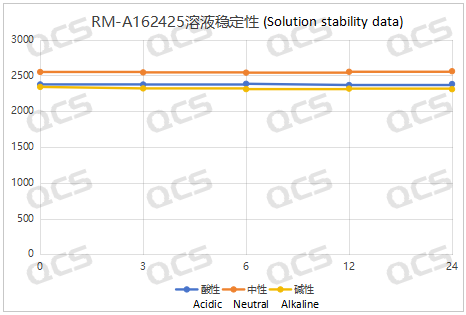
Figure 7: Summary Line Chart of Solution Stability Data for Sample RM-A162425
RM-A162480
After testing, it was found that the main peak area of sample RM-A162480 changed significantly after being placed in alkaline solution for 24 hours, with a relative standard deviation of 6.96%. So it can be considered that the sample is relatively unstable and undergoes slow degradation when placed in alkaline solution for 24 hours. The experimenter summarized and organized the sampling and testing data of the sample after being placed in acidic, neutral, and alkaline solutions for 24 hours, and created a line graph of the change in the main peak area of the sample with placement time, as shown in Figure 8. From Figure 8, it can be seen that the sample degrades rapidly after being placed in alkaline solution for 3 hours, with a decrease of about 12.29% in the area of the main peak. After being placed in alkaline solution for 3 hours, the sample no longer degrades. During the 24-hour storage of the sample in neutral and acidic solutions, there was little change in the peak area of the main peak, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that the sample is stable when placed in neutral and acidic solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-A162480 under various pH conditions are as follows:
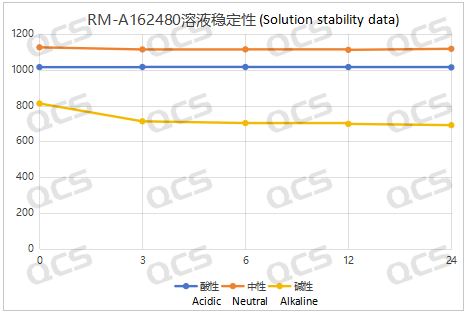
Figure 8: Summary Line Chart of Solution Stability Data for Sample RM-A162480
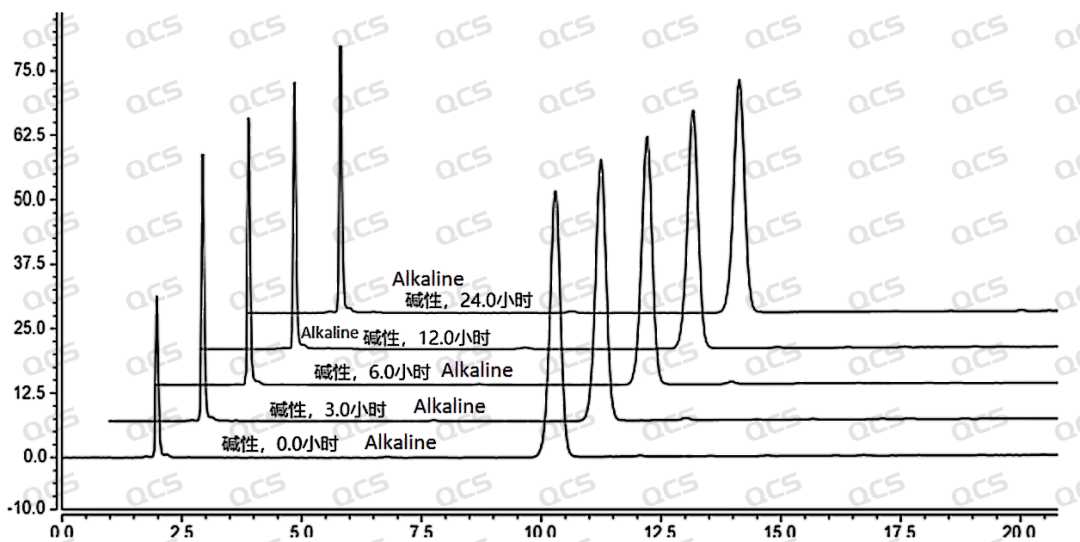
Figure 9: Stereoscopic comparison of solution stability data for sample RM-A162480
Experiment conclusion
In summary, through this experiment, we found that samples RM-A162406, RM-A162423 (BMS-778960), RM-A162424 (BMS-728626), and RM-A162425 (BMS-591329) have good stability in acidic, neutral, and alkaline solutions. Sample RM-A162480 undergoes partial degradation during the preparation process with alkaline solution, but has good stability in both neutral and acidic solutions. Sample RM-A162480 partially degrades during the preparation of alkaline solutions, but it has good stability in neutral and acidic solutions. So customers should avoid using alkaline diluents when testing sample RM-A162480, and should not touch alkali during use, storage, and transportation. If the customer has a need for the stability content of these 5 samples, welcome consult with our company.




Today, we will share a study on the stability of Antithrombotic drug-Apixaban specific impurities. Apixaban is clinically used in adult patients undergoing hip or knee replacement surgery to prevent venous thromboembolism (VTE) events.
Experimental plan
In this experiment, Our center conducted a solution stability study on five specific impurities of Apixaban using the chromatographic conditions used under the "Analysis" section of the "Apixaban" variety in the United States Pharmacopeia. The sample item number and structural formula used are shown in Figure 1 and Figure 2 below:

Figure 1: The impurity item number and structure used in this study

Figure 2: Corresponding relationship diagram between impurity codes included in the standard and impurity item numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-A162406(Apixaban Amino Acid Impurity; CAS# 2206825-87-6), RM-A162423(Apixaban Impurity Bms-778960; CAS#2098457-92-0), RM-A162424(Apixaban Impurity Bms-728626; CAS#1686149-74-5), RM-A162425(Apixaban Impurity Bms-591329; CAS#2029205-64-7) and RM-A162480(Apixaban Impurity 80; CAS#2614-10-0), and placed them in acidic, neutral, and alkaline solutions, respectively. They were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours, and then injected for testing according to the chromatographic conditions used under the "Analysis" item of the "Apixaban" variety in the United States Pharmacopeia standard. Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Experimental result
RM-A162406、RM-A162423、RM-A162424和RM-A162425
After testing, it was found that the main peak area of samples RM-A162406, RM-A162423, RM-A162424, and RM-A162425 did not change significantly during 24 hours of storage in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the above four samples are relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The main peak area data of each detection point for these four samples under different pH conditions are as follows. Among them, RM-A162406 showed a ghost peak in the detection data after being placed in neutral solution for 3 hours, which was removed:

Figure 3: Summary line chart of solution stability data of sample RM-A162406 after being placed in acidic and alkaline solutions for 24 hours

Figure 4: Summary line chart of solution stability data of sample RM-A162406 after being placed in a medium solution for 24 hours

Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-A162423

Figure 6: Summary Line Chart of Solution Stability Data for Sample RM-A162424

Figure 7: Summary Line Chart of Solution Stability Data for Sample RM-A162425
RM-A162480
After testing, it was found that the main peak area of sample RM-A162480 changed significantly after being placed in alkaline solution for 24 hours, with a relative standard deviation of 6.96%. So it can be considered that the sample is relatively unstable and undergoes slow degradation when placed in alkaline solution for 24 hours. The experimenter summarized and organized the sampling and testing data of the sample after being placed in acidic, neutral, and alkaline solutions for 24 hours, and created a line graph of the change in the main peak area of the sample with placement time, as shown in Figure 8. From Figure 8, it can be seen that the sample degrades rapidly after being placed in alkaline solution for 3 hours, with a decrease of about 12.29% in the area of the main peak. After being placed in alkaline solution for 3 hours, the sample no longer degrades. During the 24-hour storage of the sample in neutral and acidic solutions, there was little change in the peak area of the main peak, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that the sample is stable when placed in neutral and acidic solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-A162480 under various pH conditions are as follows:

Figure 8: Summary Line Chart of Solution Stability Data for Sample RM-A162480

Figure 9: Stereoscopic comparison of solution stability data for sample RM-A162480
Experiment conclusion
In summary, through this experiment, we found that samples RM-A162406, RM-A162423 (BMS-778960), RM-A162424 (BMS-728626), and RM-A162425 (BMS-591329) have good stability in acidic, neutral, and alkaline solutions. Sample RM-A162480 undergoes partial degradation during the preparation process with alkaline solution, but has good stability in both neutral and acidic solutions. Sample RM-A162480 partially degrades during the preparation of alkaline solutions, but it has good stability in neutral and acidic solutions. So customers should avoid using alkaline diluents when testing sample RM-A162480, and should not touch alkali during use, storage, and transportation. If the customer has a need for the stability content of these 5 samples, welcome consult with our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号