Time:2024-09-06


Introduction
Today, we share the study on the stability of Sitagliptin specific impurities, Sitagliptin is a new type of anti type II diabetes drug, and the first dipeptidyl peptide - Ⅳ (DPP - Ⅳ) chemobook inhibitor drug used to treat type II diabetes, often in the form of phosphonate. Sitagliptin can prevent and treat type 2 diabetes, hyperglycemia, insulin resistance, obesity, hypertension and some complications.
Experimental scheme
In this experiment, our center conducted a solution stability study on the impurities of two double bond isomers of Sitagliptin (Sitagliptin styrene acetyl analogue and Sitagliptin benzyl crotonyl analogue) using the chromatographic conditions used under the "Related substances" item of the "SITAGLIPTIN PHOSPHATE MONOHYDRATE" variety in the European Pharmacopoeia 11.0 edition. The sample numbers and structure formula are as shown in Figure 1 and Figure 2:
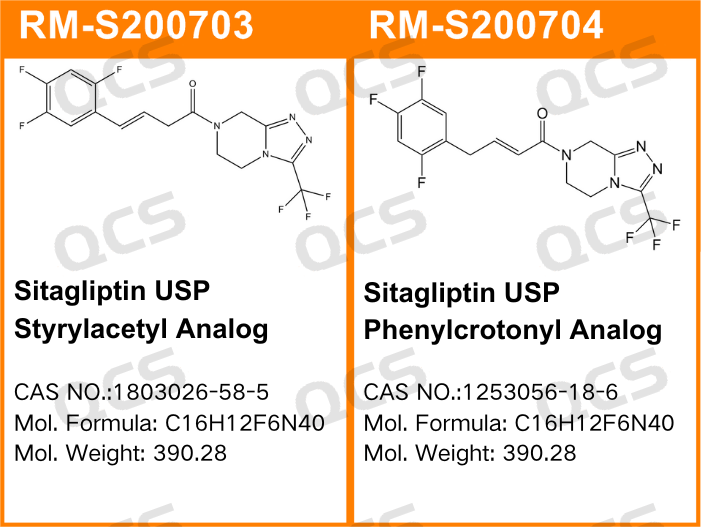
Figure 1: The impurity item number and structural formula used in this study

Figure 2: Correspondence between impurity item number and standard inclusion code
In this experiment, The experimenter will take an appropriate amount of RM-S200703 (Sitagliptin USP Styrylacetyl Analog; CAS NO: 1803026-58-5) and RM-S200704 (Sitagliptin USP Phenylrotary Analog; CAS NO: 1253056-18-6) each, place the samples separately in acidic, neutral, and alkaline solutions at room temperature and pressure for 0, 3, 6, 12, 24 hours, respectively. Follow the chromatographic conditions used under the "Related substances" section of the "SITAGLIPTIN PHOSPHATE MONOHYDRATE" variety in the European Pharmacopoeia 11.0 edition for injection detection, observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Experimental result
After testing, it was found that the main peak area of samples RM-S200703 and RM-S200704 did not change significantly when placed in acidic and neutral solutions for 24 hours, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that the two samples are relatively stable during the 24 hours in the acidic and neutral solutions. However, the area of the main peak decreased continuously while the two samples were placed in the alkaline solution for 24 hours. So the two samples are constantly degraded during the process of being placed in the alkaline solution for 24 hours. The main peak area data of sample RM-S200703 and RM-S200704 at each PH value are as follows:
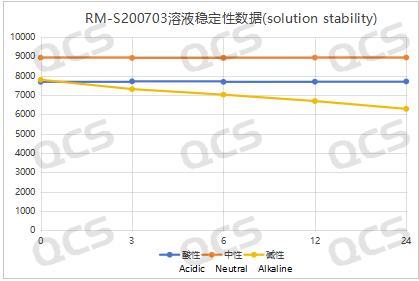
Figure 3: Summary Line Chart of Solution Stability Data for Sample RM-S200703
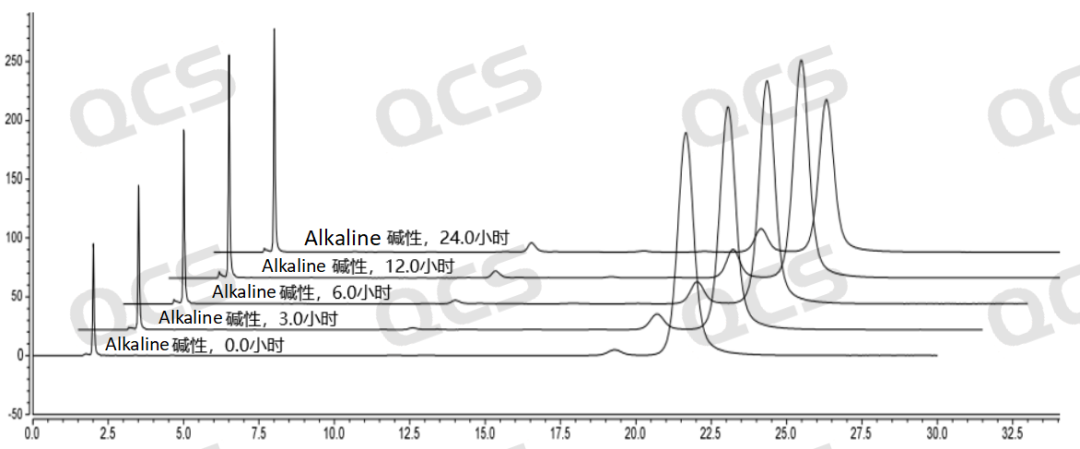
Figure 4: Summary of Solution Stability Data for Sample RM-S200703 in 3D
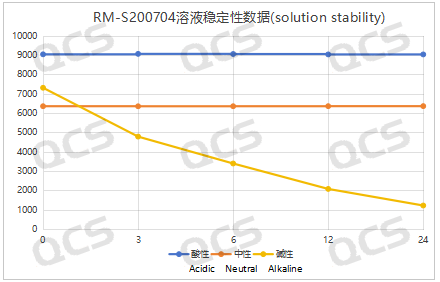
Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-S200704
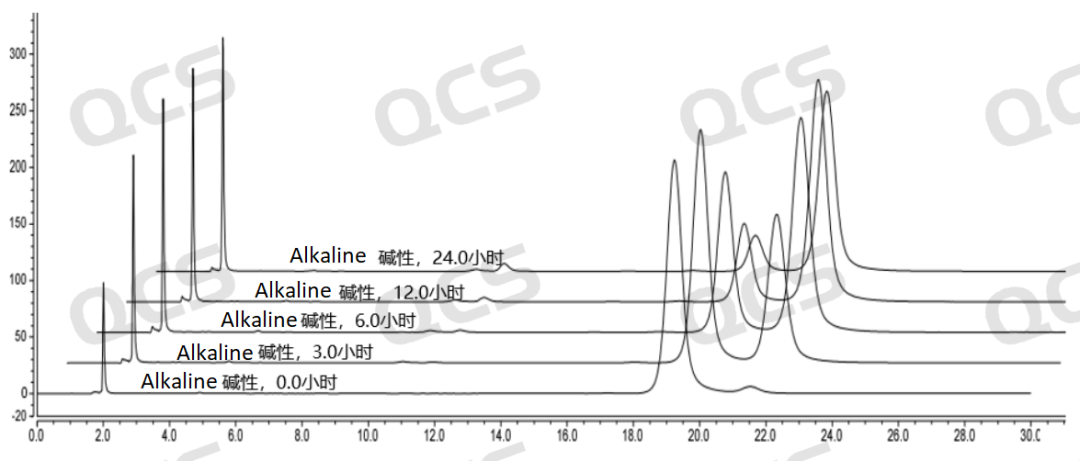
Figure 6: Summary of Solution Stability Data for Sample RM-S200704 in 3D
experiment conclusion
In summary, through this experiment, we found that samples RM-S200703 and RM-S200704 have good stability in acidic and neutral solutions, but are unstable in alkaline solutions and will continuously degrade with prolonged storage time. So customers are not allowed to touch alkali when using, transporting, and storing samples RM-S200703 and RM-S200704. If customers have a need for the stability of these two samples, welcome to consult our company.




Introduction
Today, we share the study on the stability of Sitagliptin specific impurities, Sitagliptin is a new type of anti type II diabetes drug, and the first dipeptidyl peptide - Ⅳ (DPP - Ⅳ) chemobook inhibitor drug used to treat type II diabetes, often in the form of phosphonate. Sitagliptin can prevent and treat type 2 diabetes, hyperglycemia, insulin resistance, obesity, hypertension and some complications.
Experimental scheme
In this experiment, our center conducted a solution stability study on the impurities of two double bond isomers of Sitagliptin (Sitagliptin styrene acetyl analogue and Sitagliptin benzyl crotonyl analogue) using the chromatographic conditions used under the "Related substances" item of the "SITAGLIPTIN PHOSPHATE MONOHYDRATE" variety in the European Pharmacopoeia 11.0 edition. The sample numbers and structure formula are as shown in Figure 1 and Figure 2:

Figure 1: The impurity item number and structural formula used in this study

Figure 2: Correspondence between impurity item number and standard inclusion code
In this experiment, The experimenter will take an appropriate amount of RM-S200703 (Sitagliptin USP Styrylacetyl Analog; CAS NO: 1803026-58-5) and RM-S200704 (Sitagliptin USP Phenylrotary Analog; CAS NO: 1253056-18-6) each, place the samples separately in acidic, neutral, and alkaline solutions at room temperature and pressure for 0, 3, 6, 12, 24 hours, respectively. Follow the chromatographic conditions used under the "Related substances" section of the "SITAGLIPTIN PHOSPHATE MONOHYDRATE" variety in the European Pharmacopoeia 11.0 edition for injection detection, observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Experimental result
After testing, it was found that the main peak area of samples RM-S200703 and RM-S200704 did not change significantly when placed in acidic and neutral solutions for 24 hours, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that the two samples are relatively stable during the 24 hours in the acidic and neutral solutions. However, the area of the main peak decreased continuously while the two samples were placed in the alkaline solution for 24 hours. So the two samples are constantly degraded during the process of being placed in the alkaline solution for 24 hours. The main peak area data of sample RM-S200703 and RM-S200704 at each PH value are as follows:

Figure 3: Summary Line Chart of Solution Stability Data for Sample RM-S200703

Figure 4: Summary of Solution Stability Data for Sample RM-S200703 in 3D

Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-S200704

Figure 6: Summary of Solution Stability Data for Sample RM-S200704 in 3D
experiment conclusion
In summary, through this experiment, we found that samples RM-S200703 and RM-S200704 have good stability in acidic and neutral solutions, but are unstable in alkaline solutions and will continuously degrade with prolonged storage time. So customers are not allowed to touch alkali when using, transporting, and storing samples RM-S200703 and RM-S200704. If customers have a need for the stability of these two samples, welcome to consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号