Time:2024-08-30
The chemical structure of impurity AML-2 in the imported drug registration standard Bisoprolol and Amlodipine Tablets - JX20210038 is erroneous. QCS can provide the accurate AML-2 impurity, with a retention time that aligns with the RRT result specified in the standard.
Introduction
Based on publicly available information, it is known that there are currently two companies, Yuan Dong (Chengdu Shuo De) and Shijiazhuang Siyu Pharmaceutical, which have obtained marketing authorization for bisoprolol amlodipine tablets. In addition, more than ten pharmaceutical companies including Shandong Xin Shi Ji Pharmaceutical, Beijing Fu Yuan Pharmaceutical, Zhejiang Hua Yuan Pharmaceutical, TianDa Pharmaceutical (Zhuhai), Shandong Qi Du Pharmaceutical, and Jiangxi Shi Mei Pharmaceutical have submitted applications for the market approval of bisoprolol amlodipine tablets under new registration classifications. These applications are all in the process of undergoing review and approval.
The Cause of the Incident
The AML-2 impurity is a specified impurity in the import drug registration standard for Bisoprolol and Amlodipine Tablets - JX20210038. The standard specifies its structural formula as: N-(2-{[4-(2-chlorophenyl)-3-(ethoxycarbonyl)5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine-2-yl]methoxy}ethyl)aspartic acid (depicted on the left in Figure 1), which corresponds to QCS standard reference material number RM-A131257 (depicted on the right in Figure 1).
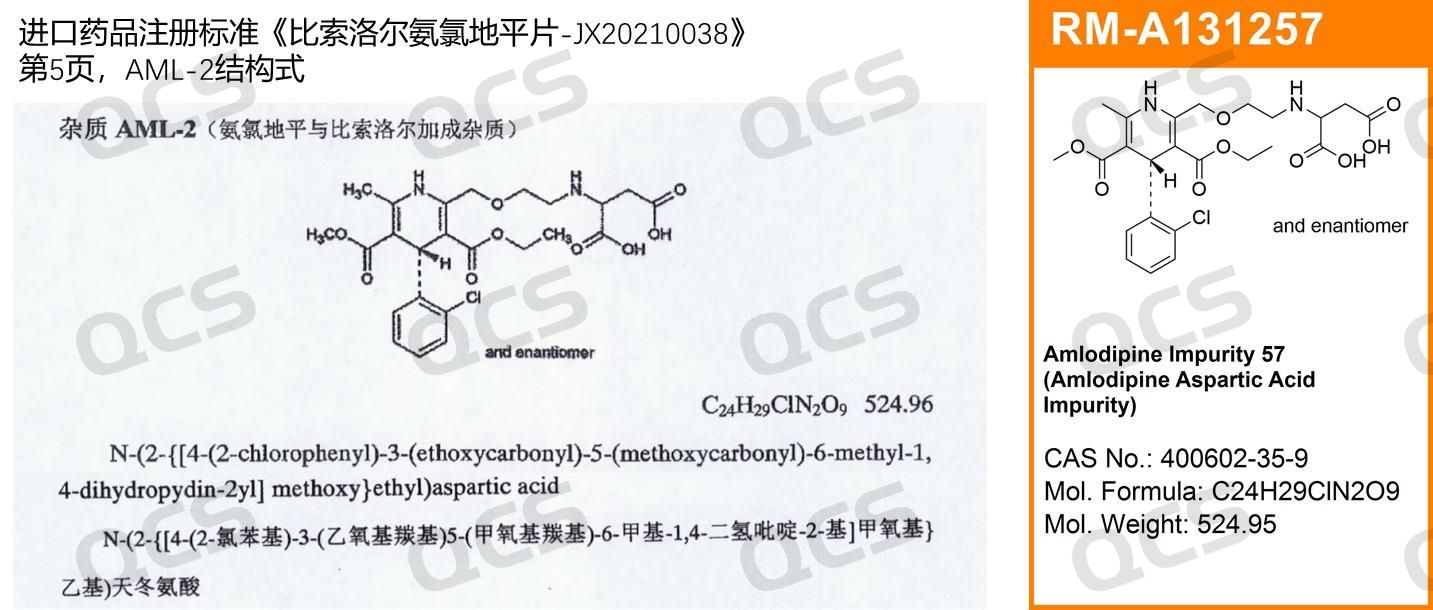
Figure 1: Structure of AML-2 as claimed in the registration standard for imported drugs
We have recently received customer feedback regarding the use of the specified structure N-(2-{[4-(2-chlorophenyl)-3-(ethoxycarbonyl)5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridin-2-yl]methoxy}ethyl)aspartic acid (CAT No: RM-A131257; Amlodipine Impurity 57 (Amlodipine Aspartic Acid Impurity); CAS NO: 400602-35-9) during the study of AML-2 in samples. Anomalies were observed in the results, as the structure (CAT No: RM-A131257) did not correspond to the typical chromatographic peak of AML-2 under standard liquid chromatography conditions.
Based on customer feedback, the system suitability assessment was conducted using standard substances claimed in the import standard structure. With the exception of bisoprolol amlodipine additive impurity AML-2, the peak order and relative retention time of the remaining five impurities were found to be essentially consistent with those specified in the import registration standard. However, it was observed that for impurity AML-2, its relative retention time in the standard should be 1.27, while the measured value for this structural sample was approximately 1.0 (with the sample peak almost overlapping with the API peak). Even after changing to a different chromatographic column for testing, there were no significant changes in chromatographic results—except for AML-2, where both peak order and relative retention time of other impurities remained consistent with those specified in the import registration standard. The abnormal elution behavior of AML-2 is currently under investigation (Figure 2 shows detection data feedback from the customer).
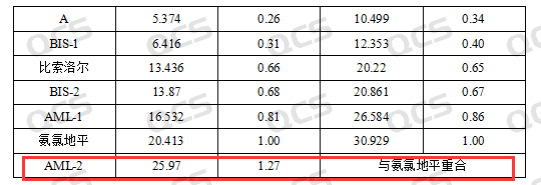
Figure 2: Detected impurities data for each impurity according to the customer's standard method (only AML-2 data is abnormal)
Experimental Scheme
The AML-2 structure claimed in the import registration standard is a complex of amlodipine and fumaric acid (refer to Figure 1). Therefore, we conducted a review of the detection data for the product corresponding to this structure (CAT No: RM-A131257), and its nuclear magnetic resonance (NMR) and mass spectrometry data were found to be consistent with the target structure, confirming the accuracy of the structure (product data are shown in Figure 3).
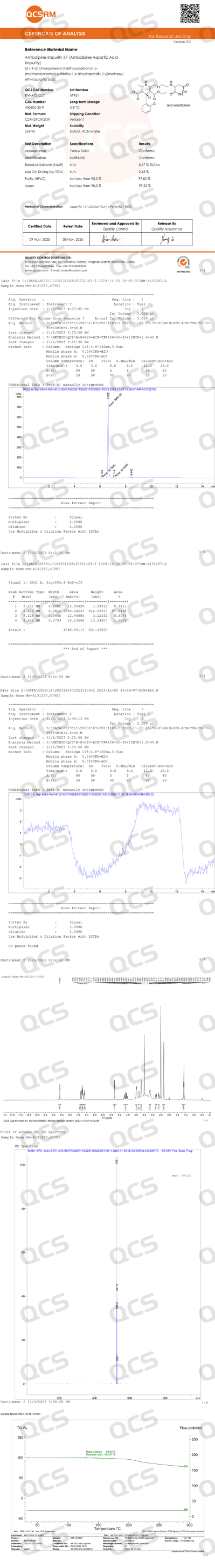
Figure 3: Test data and report for the nominal structure of the AML-2 product
After confirming the product structure was correct, the QCS R&D Center re-examined the liquid chromatography results provided by the customer. However, when we used the impurity method specified in the import registration standard (Bisoprolol Amlodipine Tablets JX20210038) to test for AML-2, we got the same results as the customer - AML-2 did not appear in the RRT 1.2 range as specified in the standard, but was very close to the retention time of amlodipine (Figure 4 shows the standard inclusion description of AML-2, and Figure 5 shows the chromatogram data of AML-2 tested according to the standard method)
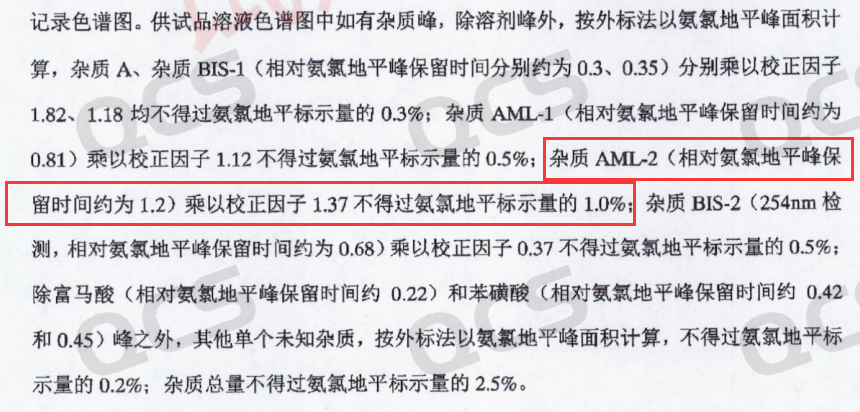
Figure 4: Standardized representation of the RRT and adjustment factors for AML-2
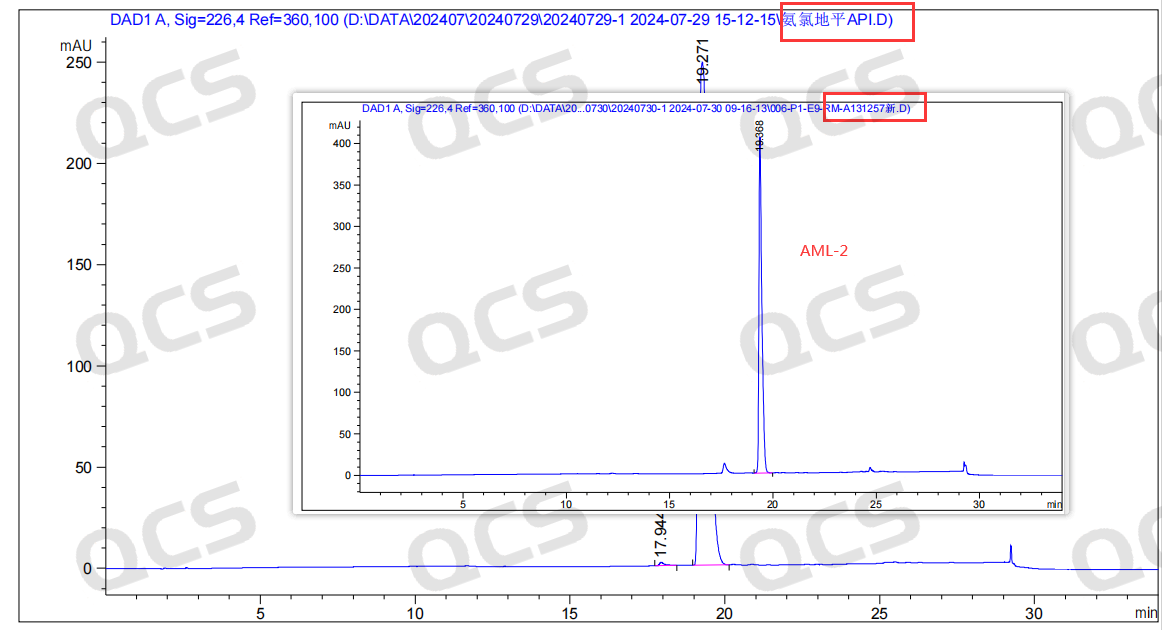
Figure 5: Chromatogram illustrating the detection of amlodipine and its AML-2 impurity by our laboratory under standardized conditions.
The data revealed that the retention time (RT) of amlodipine active pharmaceutical ingredient (API) was 19.27 minutes, while the RT of the existing so-called AML-2 samples was 19.37 minutes, indicating a close similarity to that of the API. Moreover, despite testing various chromatographic columns, the optimal separation achieved in liquid chromatography resulted in a relative retention time (RRT) of only 1.02, which is significantly lower than the RRT value of 1.2 specified in the standard.
The results obtained from liquid chromatography using this sample did not align with those specified in the import registration standard. However, the QCS Standard Material Research and Development Center has verified that the sample is consistent with the structural representation outlined in the standard. This raises a pertinent question: could it be that the declared structural formula of AML-2 in the import registration standard is incorrect?
To clarify: the Bisoprolol-amlodipine compound contains an impurity designated as AML-2, which exhibits a relative retention time of 1.27. However, this impurity is not N-(2-{[4-(2-chlorophenyl)-3-(ethoxycarbonyl)-5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine-2-yl]methoxy}ethyl)aspartic acid (CAT No: RM-A131257). The authentic AML-2 impurity possesses its own distinct structural formula.
So, who is the AML-2 included in the standard?
Fortunately, we have identified the true structure of the AML-2 impurity!
Research and Development Process
Based on customer data feedback and our proprietary research on the existing AML-2, we conducted extensive investigations over several months. Ultimately, we successfully identified the authentic AML-2 among hundreds of test samples and established a definitive standard for it.
The journey of investigating the unknown can often be monotonous and laborious; however, upon discovering the mechanisms behind product production and validating our concepts, the sense of enlightenment is truly beyond words. After nearly two months of diligent exploration, we have successfully developed the AML-2 product.
Based on the newly synthesized AML-2 standard sample, which is a mixture of amlodipine and AML-1, the peak order observed in the authentic AML-2 structural sample aligns with the import registration standard. The calculated relative retention time (RRT) for both AML-2 and amlodipine is 1.27 (24.678/19.412=1.27), consistent with the RRT specified in the standard.
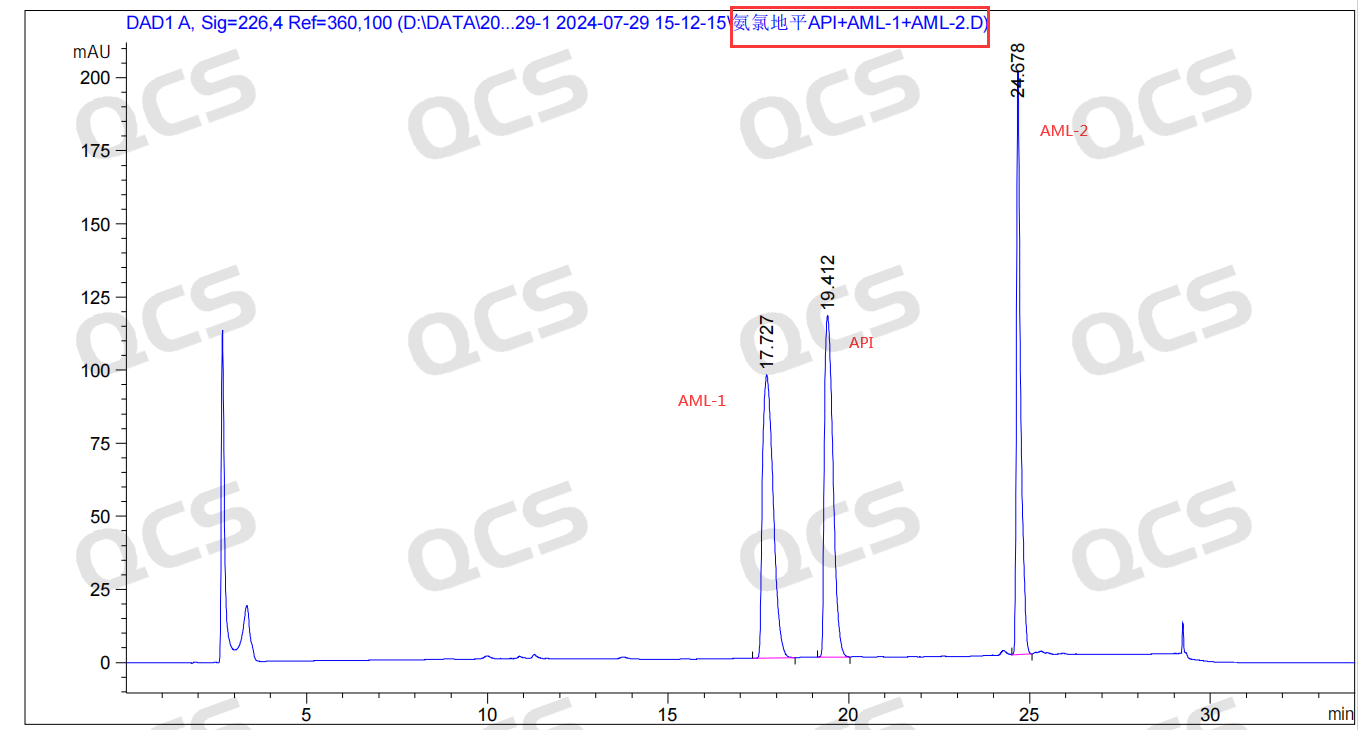
Figure 6: Data on the combination of AML-1 and AML-2 with amlodipine injection.
Experimental Conclusion
Based on the comparative analysis of the retention times for both authentic and counterfeit AML-2 and API, we have substantial grounds to question the accuracy of the structure of AML-2 as specified in the import registration standard (Bisoprolol Amlodipine Tablet JX20210038), suggesting it may not be correct but rather indicative of an alternative entity.
In conclusion, our verification experiment revealed a discrepancy between the structure specified in the import standard and that observed in the chromatogram. This issue has been raised by all customers involved in this project. We encourage customers to request a sample for verifying the accuracy of AML-2.


The chemical structure of impurity AML-2 in the imported drug registration standard Bisoprolol and Amlodipine Tablets - JX20210038 is erroneous. QCS can provide the accurate AML-2 impurity, with a retention time that aligns with the RRT result specified in the standard.
Introduction
Based on publicly available information, it is known that there are currently two companies, Yuan Dong (Chengdu Shuo De) and Shijiazhuang Siyu Pharmaceutical, which have obtained marketing authorization for bisoprolol amlodipine tablets. In addition, more than ten pharmaceutical companies including Shandong Xin Shi Ji Pharmaceutical, Beijing Fu Yuan Pharmaceutical, Zhejiang Hua Yuan Pharmaceutical, TianDa Pharmaceutical (Zhuhai), Shandong Qi Du Pharmaceutical, and Jiangxi Shi Mei Pharmaceutical have submitted applications for the market approval of bisoprolol amlodipine tablets under new registration classifications. These applications are all in the process of undergoing review and approval.
The Cause of the Incident
The AML-2 impurity is a specified impurity in the import drug registration standard for Bisoprolol and Amlodipine Tablets - JX20210038. The standard specifies its structural formula as: N-(2-{[4-(2-chlorophenyl)-3-(ethoxycarbonyl)5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine-2-yl]methoxy}ethyl)aspartic acid (depicted on the left in Figure 1), which corresponds to QCS standard reference material number RM-A131257 (depicted on the right in Figure 1).

Figure 1: Structure of AML-2 as claimed in the registration standard for imported drugs
We have recently received customer feedback regarding the use of the specified structure N-(2-{[4-(2-chlorophenyl)-3-(ethoxycarbonyl)5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridin-2-yl]methoxy}ethyl)aspartic acid (CAT No: RM-A131257; Amlodipine Impurity 57 (Amlodipine Aspartic Acid Impurity); CAS NO: 400602-35-9) during the study of AML-2 in samples. Anomalies were observed in the results, as the structure (CAT No: RM-A131257) did not correspond to the typical chromatographic peak of AML-2 under standard liquid chromatography conditions.
Based on customer feedback, the system suitability assessment was conducted using standard substances claimed in the import standard structure. With the exception of bisoprolol amlodipine additive impurity AML-2, the peak order and relative retention time of the remaining five impurities were found to be essentially consistent with those specified in the import registration standard. However, it was observed that for impurity AML-2, its relative retention time in the standard should be 1.27, while the measured value for this structural sample was approximately 1.0 (with the sample peak almost overlapping with the API peak). Even after changing to a different chromatographic column for testing, there were no significant changes in chromatographic results—except for AML-2, where both peak order and relative retention time of other impurities remained consistent with those specified in the import registration standard. The abnormal elution behavior of AML-2 is currently under investigation (Figure 2 shows detection data feedback from the customer).

Figure 2: Detected impurities data for each impurity according to the customer's standard method (only AML-2 data is abnormal)
Experimental Scheme
The AML-2 structure claimed in the import registration standard is a complex of amlodipine and fumaric acid (refer to Figure 1). Therefore, we conducted a review of the detection data for the product corresponding to this structure (CAT No: RM-A131257), and its nuclear magnetic resonance (NMR) and mass spectrometry data were found to be consistent with the target structure, confirming the accuracy of the structure (product data are shown in Figure 3).

Figure 3: Test data and report for the nominal structure of the AML-2 product
After confirming the product structure was correct, the QCS R&D Center re-examined the liquid chromatography results provided by the customer. However, when we used the impurity method specified in the import registration standard (Bisoprolol Amlodipine Tablets JX20210038) to test for AML-2, we got the same results as the customer - AML-2 did not appear in the RRT 1.2 range as specified in the standard, but was very close to the retention time of amlodipine (Figure 4 shows the standard inclusion description of AML-2, and Figure 5 shows the chromatogram data of AML-2 tested according to the standard method)

Figure 4: Standardized representation of the RRT and adjustment factors for AML-2

Figure 5: Chromatogram illustrating the detection of amlodipine and its AML-2 impurity by our laboratory under standardized conditions.
The data revealed that the retention time (RT) of amlodipine active pharmaceutical ingredient (API) was 19.27 minutes, while the RT of the existing so-called AML-2 samples was 19.37 minutes, indicating a close similarity to that of the API. Moreover, despite testing various chromatographic columns, the optimal separation achieved in liquid chromatography resulted in a relative retention time (RRT) of only 1.02, which is significantly lower than the RRT value of 1.2 specified in the standard.
The results obtained from liquid chromatography using this sample did not align with those specified in the import registration standard. However, the QCS Standard Material Research and Development Center has verified that the sample is consistent with the structural representation outlined in the standard. This raises a pertinent question: could it be that the declared structural formula of AML-2 in the import registration standard is incorrect?
To clarify: the Bisoprolol-amlodipine compound contains an impurity designated as AML-2, which exhibits a relative retention time of 1.27. However, this impurity is not N-(2-{[4-(2-chlorophenyl)-3-(ethoxycarbonyl)-5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine-2-yl]methoxy}ethyl)aspartic acid (CAT No: RM-A131257). The authentic AML-2 impurity possesses its own distinct structural formula.
So, who is the AML-2 included in the standard?
Fortunately, we have identified the true structure of the AML-2 impurity!
Research and Development Process
Based on customer data feedback and our proprietary research on the existing AML-2, we conducted extensive investigations over several months. Ultimately, we successfully identified the authentic AML-2 among hundreds of test samples and established a definitive standard for it.
The journey of investigating the unknown can often be monotonous and laborious; however, upon discovering the mechanisms behind product production and validating our concepts, the sense of enlightenment is truly beyond words. After nearly two months of diligent exploration, we have successfully developed the AML-2 product.
Based on the newly synthesized AML-2 standard sample, which is a mixture of amlodipine and AML-1, the peak order observed in the authentic AML-2 structural sample aligns with the import registration standard. The calculated relative retention time (RRT) for both AML-2 and amlodipine is 1.27 (24.678/19.412=1.27), consistent with the RRT specified in the standard.

Figure 6: Data on the combination of AML-1 and AML-2 with amlodipine injection.
Experimental Conclusion
Based on the comparative analysis of the retention times for both authentic and counterfeit AML-2 and API, we have substantial grounds to question the accuracy of the structure of AML-2 as specified in the import registration standard (Bisoprolol Amlodipine Tablet JX20210038), suggesting it may not be correct but rather indicative of an alternative entity.
In conclusion, our verification experiment revealed a discrepancy between the structure specified in the import standard and that observed in the chromatogram. This issue has been raised by all customers involved in this project. We encourage customers to request a sample for verifying the accuracy of AML-2.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号