Time:2024-08-23
Introduction
Today, we will share the research on the stability of brain metabolic activator Citicoline specific impurities. Citicoline is a brain metabolic activator that can promote respiration of brain cells, improve brain function, enhance the function of the ascending reticular formation activation system, promote awakening, and reduce cerebral vascular resistance.
Experimental scheme
In this experiment, our center conducted a solution stability study on two specific impurities of Citicoline using the chromatographic conditions used under the "RELATED COMPOUNDS" item of the "Citicoline" variety in the USP-NF2024 edition of the United States Pharmacopeia. The sample item number and structural formula used are shown in Figure 1 and Figure 2:
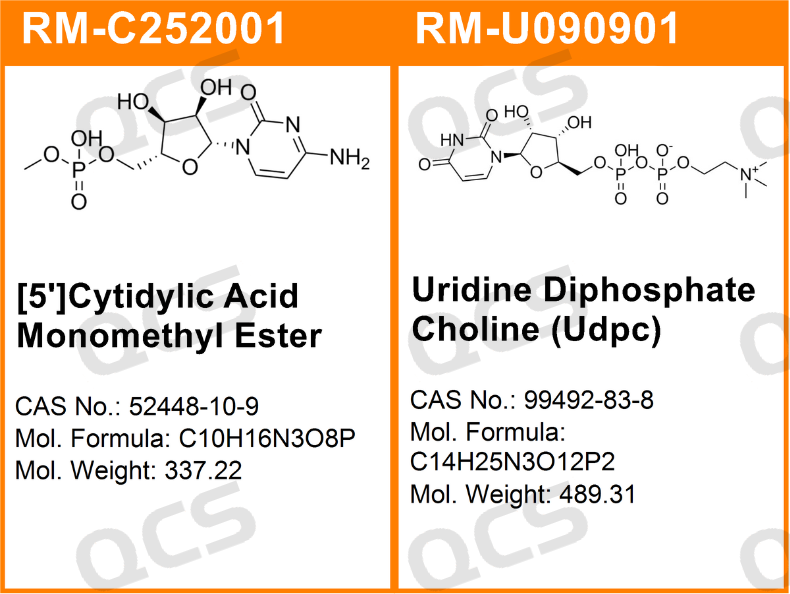
Figure 1: The impurity item number and structural formula used in this study
Research on Specific Impurities
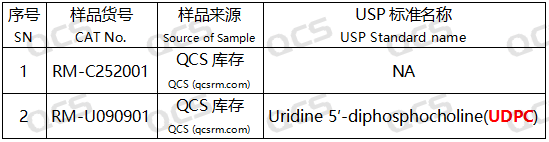
Figure 2: Corresponding relationship diagram between impurity codes included in the standard and impurity item numbers used in this study
In this experiment, the experimenter placed appropriate amounts of RM-C252001 ([5 '] Cytidylic Acid Monomethyl Ester; CAS: 52448-10-9) and RM-U090901 (Uridine Diphosphate Choline [UDPC]; CAS: 99492-83-8) in acidic, neutral, and alkaline solutions, respectively, at room temperature and pressure for 0, 3, 6, 12, and 24 hours, and then injected the samples for detection according to the chromatographic conditions used under the "RELATED COMPOUNDS" item of the "Citicoline" variety in the USP-NF2024 edition of the United States Pharmacopeia. The chromatograms were observed as the sample solution was left for an extended period of time. The change in peak area of the main peak is used as a basis to determine the solution stability of the sample.
Empirical conclusion
After testing, it was found that the main peak area of samples RM-C252001 and RM-U090901 (UDPC) did not change significantly during 24 hours of storage in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the two samples are relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The main peak area data of each detection point under various pH conditions of the sample are as follows:
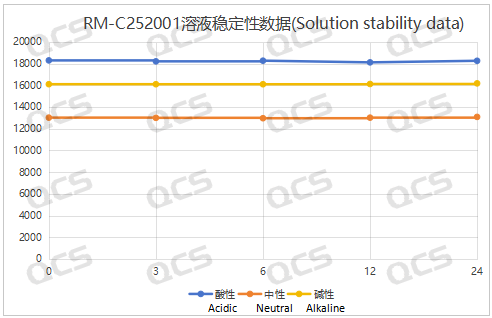
Figure 3: Summary line chart of solution stability data for sample RM-C252001
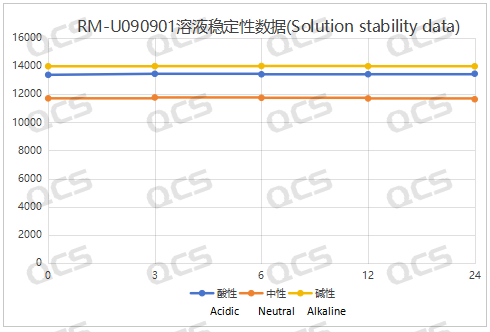
Figure 4: Summary line chart of solution stability data for sample RM-U090901 (UDPC)
In summary, through this stability experiment, we found that samples RM-C252001 and RM-U090901 (UDPC) have good stability under acid-base neutral conditions. The only thing to note is that RM-U090901 (UDPC) has strong hygroscopicity, so more attention should be paid during weighing and transfer. Firstly, if possible, try to weigh in a glove box; The second is that the weighing process should be as fast as possible; Thirdly, this product is not suitable for repeated weighing, and it is recommended to purchase small packaging as much as possible. If customers have a need for the stability of these two samples, welcome to consult our company.


Introduction
Today, we will share the research on the stability of brain metabolic activator Citicoline specific impurities. Citicoline is a brain metabolic activator that can promote respiration of brain cells, improve brain function, enhance the function of the ascending reticular formation activation system, promote awakening, and reduce cerebral vascular resistance.
Experimental scheme
In this experiment, our center conducted a solution stability study on two specific impurities of Citicoline using the chromatographic conditions used under the "RELATED COMPOUNDS" item of the "Citicoline" variety in the USP-NF2024 edition of the United States Pharmacopeia. The sample item number and structural formula used are shown in Figure 1 and Figure 2:

Figure 1: The impurity item number and structural formula used in this study
Research on Specific Impurities

Figure 2: Corresponding relationship diagram between impurity codes included in the standard and impurity item numbers used in this study
In this experiment, the experimenter placed appropriate amounts of RM-C252001 ([5 '] Cytidylic Acid Monomethyl Ester; CAS: 52448-10-9) and RM-U090901 (Uridine Diphosphate Choline [UDPC]; CAS: 99492-83-8) in acidic, neutral, and alkaline solutions, respectively, at room temperature and pressure for 0, 3, 6, 12, and 24 hours, and then injected the samples for detection according to the chromatographic conditions used under the "RELATED COMPOUNDS" item of the "Citicoline" variety in the USP-NF2024 edition of the United States Pharmacopeia. The chromatograms were observed as the sample solution was left for an extended period of time. The change in peak area of the main peak is used as a basis to determine the solution stability of the sample.
Empirical conclusion
After testing, it was found that the main peak area of samples RM-C252001 and RM-U090901 (UDPC) did not change significantly during 24 hours of storage in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the two samples are relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The main peak area data of each detection point under various pH conditions of the sample are as follows:

Figure 3: Summary line chart of solution stability data for sample RM-C252001

Figure 4: Summary line chart of solution stability data for sample RM-U090901 (UDPC)
In summary, through this stability experiment, we found that samples RM-C252001 and RM-U090901 (UDPC) have good stability under acid-base neutral conditions. The only thing to note is that RM-U090901 (UDPC) has strong hygroscopicity, so more attention should be paid during weighing and transfer. Firstly, if possible, try to weigh in a glove box; The second is that the weighing process should be as fast as possible; Thirdly, this product is not suitable for repeated weighing, and it is recommended to purchase small packaging as much as possible. If customers have a need for the stability of these two samples, welcome to consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号