Time:2024-08-09
introduction
Following the qualitative study we published last year on "Sharing research on related impurities of Bilastine tablets for the Treatment of Rhinitis and Urticaria", today our center will continue to share the stability study of Bilastine specific impurities.
experimental scheme
In this experiment, our center conducted a study on the solubility stability of three specific impurities in the imported drug registration standard "Bilastine Tablets" (standard number JX20130329) issued by the State Food and Drug Administration, comparing the chromatographic conditions used under the "related substances" inspection item with three specific impurities in Bilastine. The sample item numbers and structural formulas used are shown in Figure 1 and Figure 2:
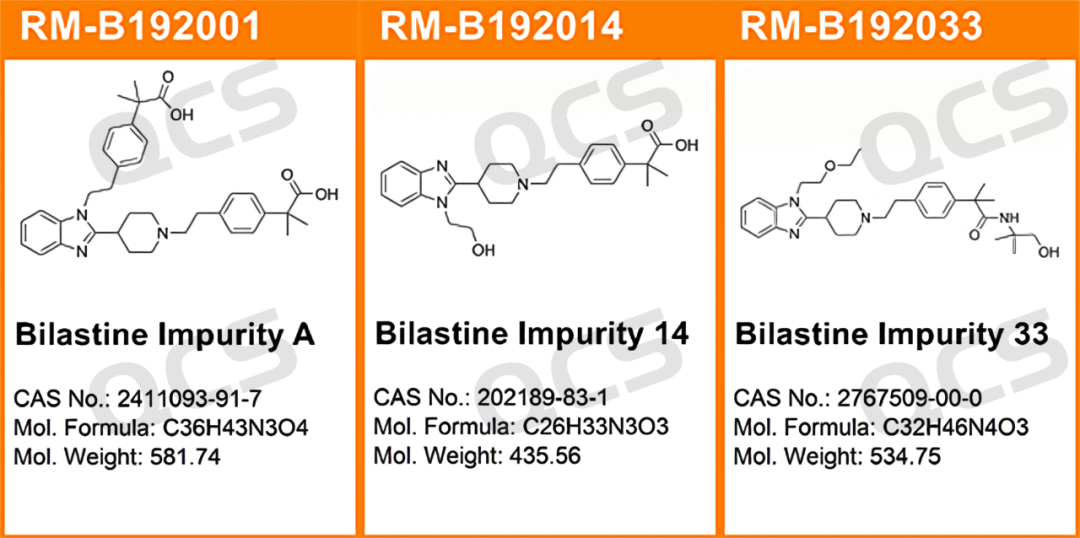
Figure 1: The impurity QCS item number and structure used in this study
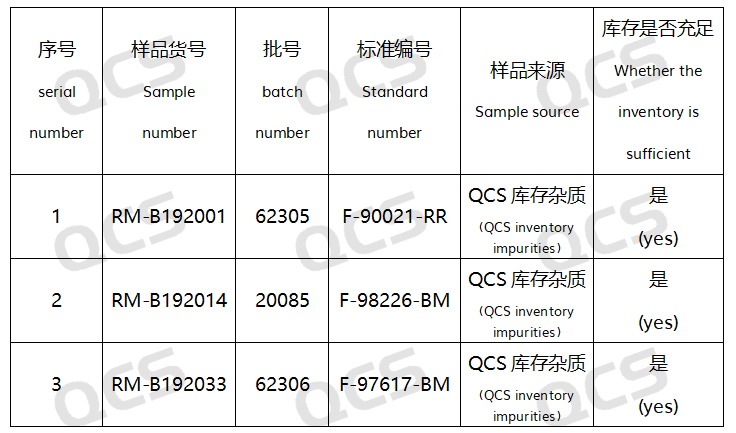
Figure 2: Correspondence between Import Standard Impurity Code and QCS Item Number
In this experiment, The experimenter took appropriate amounts of RM-B192001(F-90021-RR; Bilastine Impurity A; CAS#2411093-91-7), RM-B192014(F-98226-BM; Bilastine Impurity 14; CAS#202189-83-1) and RM-B192033(F-97617-BM; Bilastine Impurity 33; CAS#2767509-00-0), then placed them in acidic, neutral and alkaline solutions, respectively, At room temperature and pressure for 0, 3, 6, 12, and 24 hours, follow the chromatographic conditions used under the "Related Substances" inspection item in the "Bilastine Tablets" Import Drug Registration Standard (Standard No. JX20130329) for injection detection. Observe the changes in the peak area of the main peak of the sample as the sample is placed for an extended period of time. Based on this, determine the stability of the sample solution
Empirical conclusion
RM-B192001(F-90021-RR)
After testing, it was found that the main peak area of sample RM-B192001 (F-90021-RR) did not change significantly after being placed in acidic and neutral solutions for 24 hours, with a relative standard deviation of less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and neutral solutions for 24 hours. However, during the process of placing the sample in alkaline solution for 24 hours, the area of the main peak continuously decreased. Comparing the spectra obtained by placing the sample in alkaline solution for 0.0 hours and in neutral solution for 0.0 hours, it can be found that the sample has already undergone partial degradation during the preparation of the sample solution with alkaline solution, and continued to degrade slowly during the process of placing it in alkaline solution from 0.0 hours to 24.0 hours The peak area data of the main peak at each detection point of sample RM-B192001 (F-90021-RR) under various pH conditions are as follows:
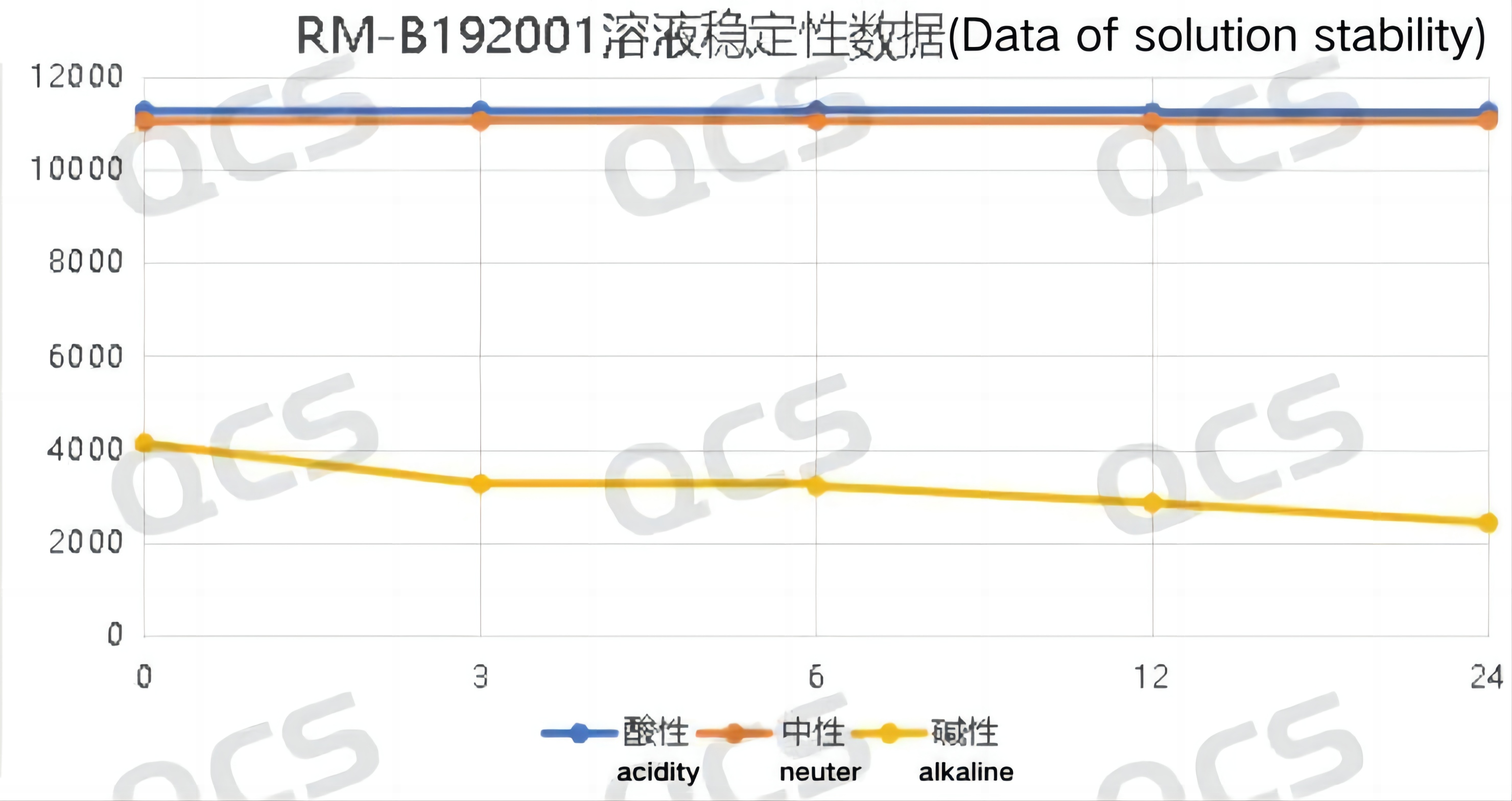
Figure 3: Summary of Solution Stability Data for Sample RM-B192001 (F-90021-RR)
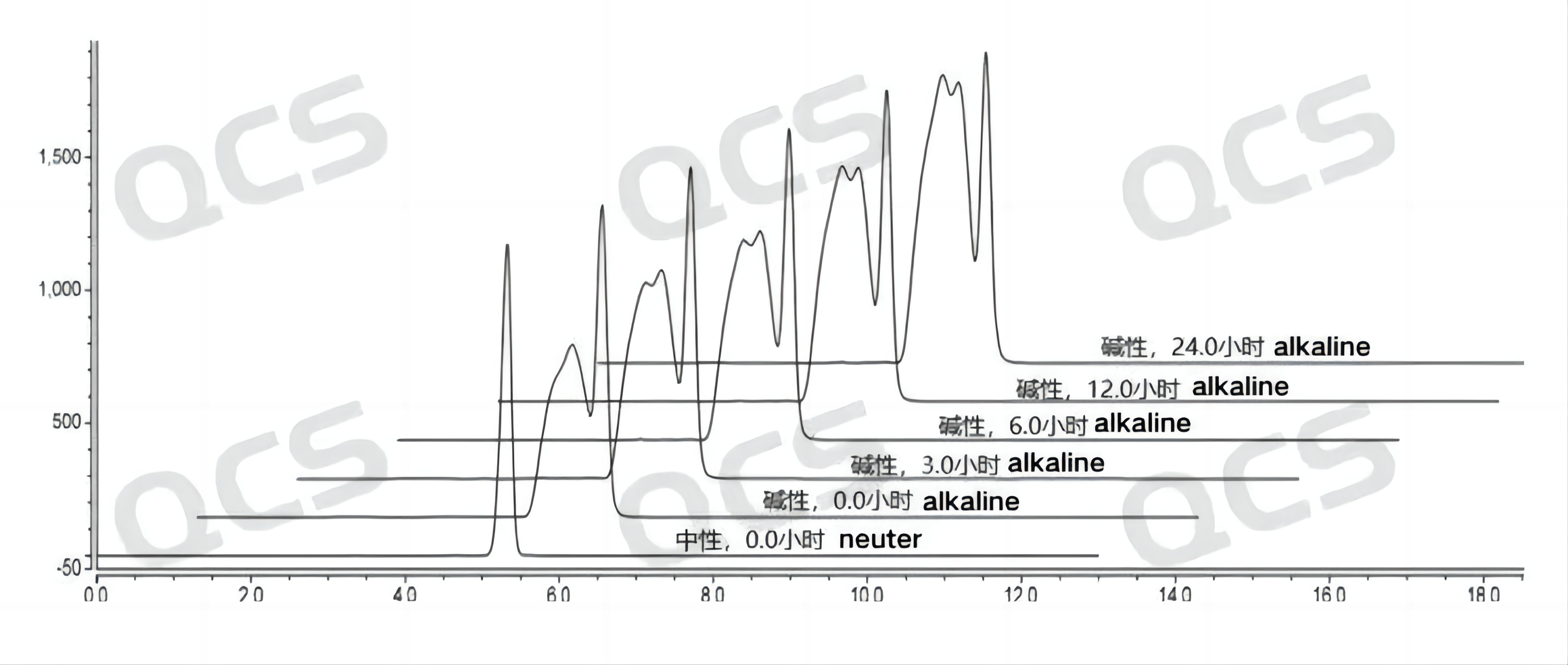
Figure 4: Summary of solution stability data for sample RM-B192001 (F-90021-RR)
RM-B192014(F-98226-BM)
After testing, it was found that the main peak area of sample RM-B192014 (F-98226-BM) did not change significantly during 24 hours of storage in acidic and neutral solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and neutral solutions for 24 hours. However, the sample appears to be less stable in alkaline solution, and interesting "abnormal" experimental results were observed during alkaline stability testing of the sample.
When the sample was placed in an alkaline solution and immediately tested on the machine (at 0.0 hours in the figure), the obtained spectrum was compared with that obtained under neutral solution conditions. It was found that the retention time of the main peak of the spectrum obtained by the sample in alkaline solution changed significantly from that under neutral conditions, but the result did not further "deteriorate" with the extension of time. Moreover, after placing the sample in an alkaline solution for 24 hours and adjusting its pH to neutral, the retention time of the main peak of the sample was restored to be consistent with the results obtained under neutral solution conditions. The above results indicate that the "abnormal" results observed in sample RM-B192014 (F-98226-BM) under alkaline conditions are more likely to be due to changes in the ion state of the sample under this pH condition rather than sample degradation. The sample results can be restored with pH adjustment. Therefore, it can be considered that the sample is relatively stable when placed in an alkaline solution for 24 hours. The main peak peak area data of sample RM-B192014 (F-98226-BM) at each PH value is as follows:
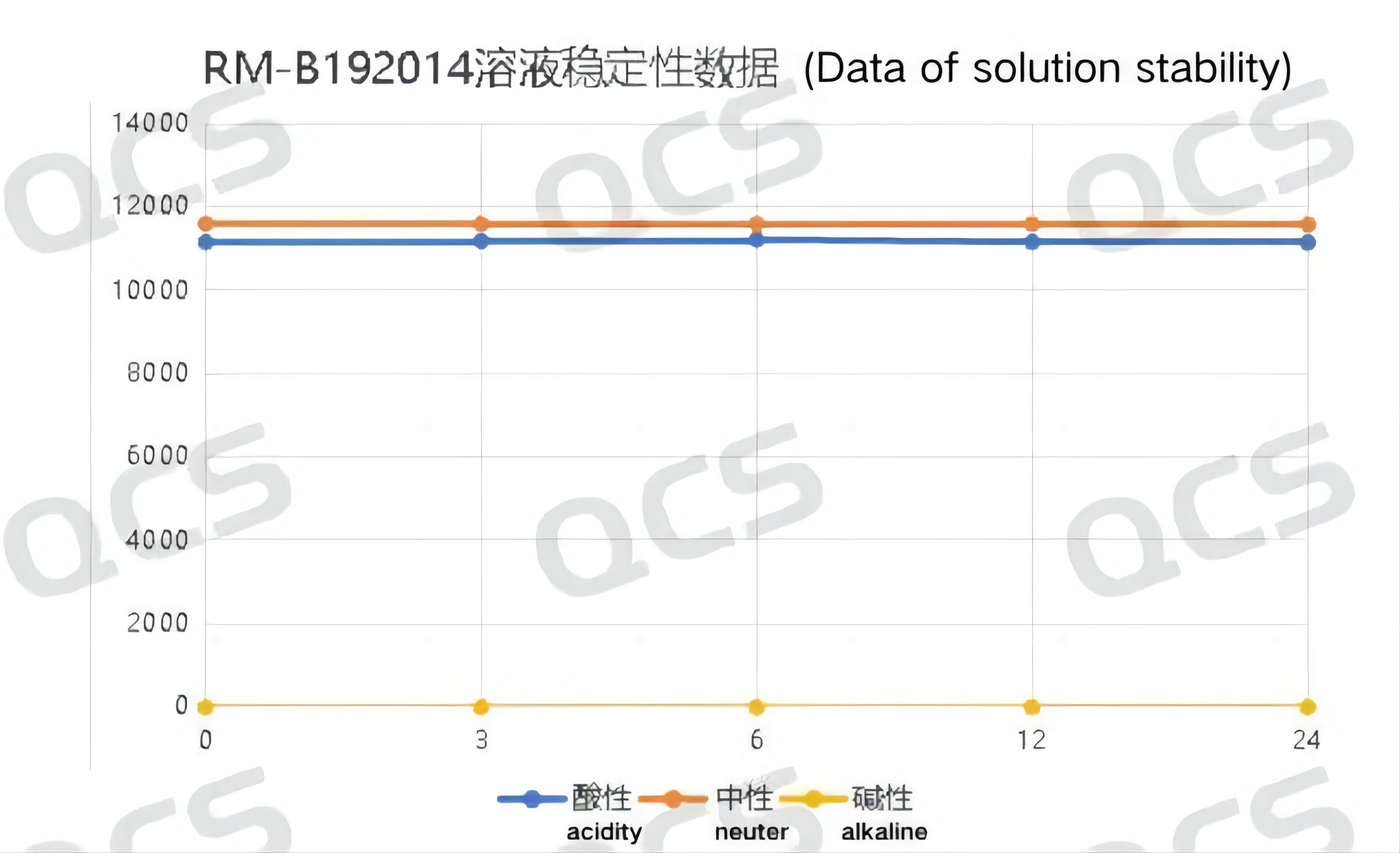
Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-B192014 (F-98226-BM)
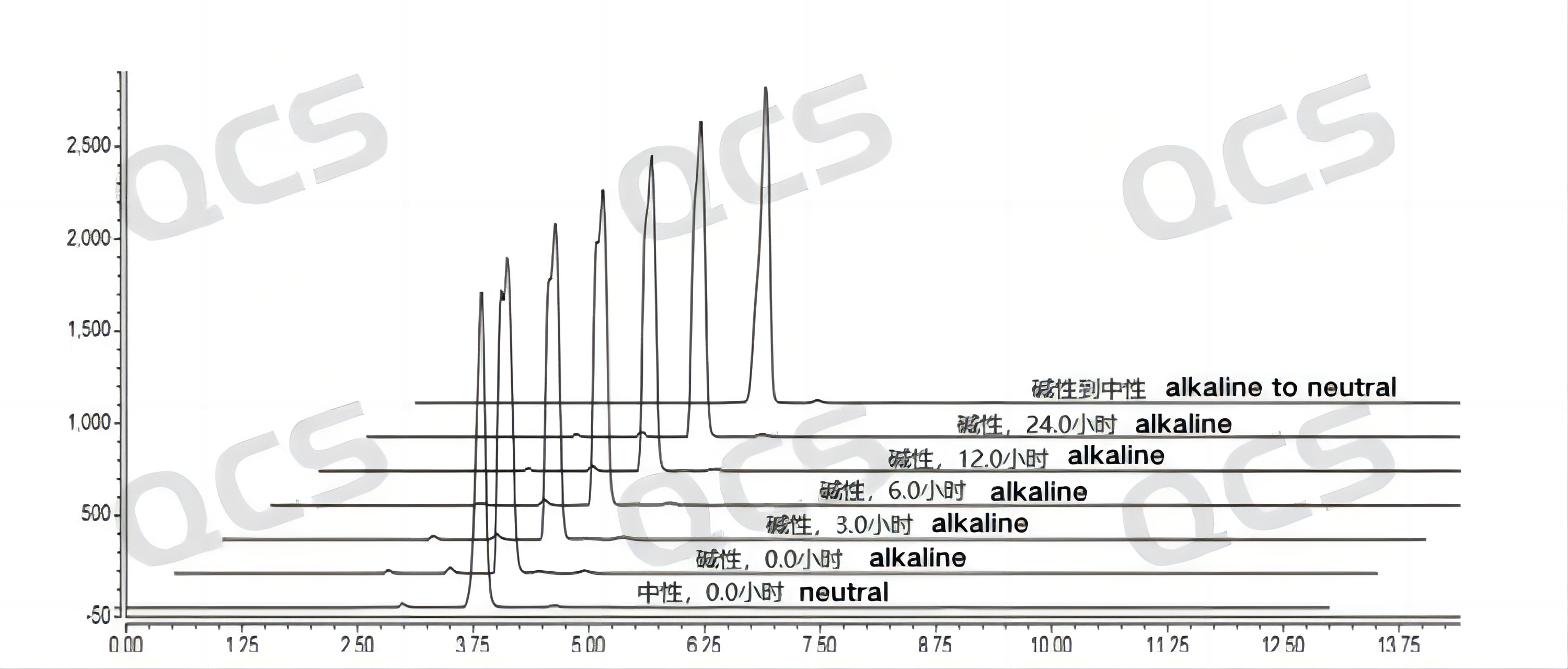
Figure 6: Summary of Solution Stability Data for Sample RM-B192014 (F-98226-BM)
RM-B192033(F-97617-BM)
After testing, it was found that the main peak area of sample RM-B192033 (F-97617-BM) did not change significantly during 24 hours of storage in alkaline and neutral solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in neutral and alkaline solutions for 24 hours. However, the main peak area of the sample changed significantly after being placed in an acidic solution for 24 hours. Comparing the spectra obtained by placing the sample in an acidic solution for 0.0 hours with those obtained by placing it in a neutral solution for 0.0 hours, it can be found that the sample was completely degraded during the preparation of the sample solution with acidic solution. The peak area data of the main peak at each detection point of sample RM-B192033 (F-97617-BM) under various pH conditions are as follows:
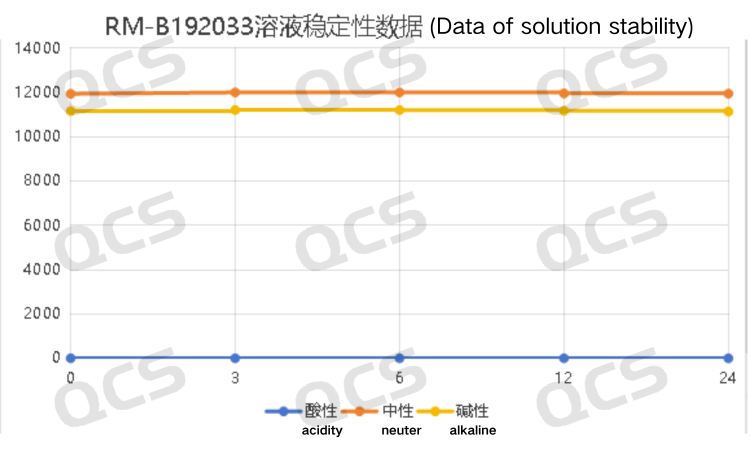
Figure 7: Summary Line Chart of Solution Stability Data for Sample RM-B192033 (F-97617-BM)
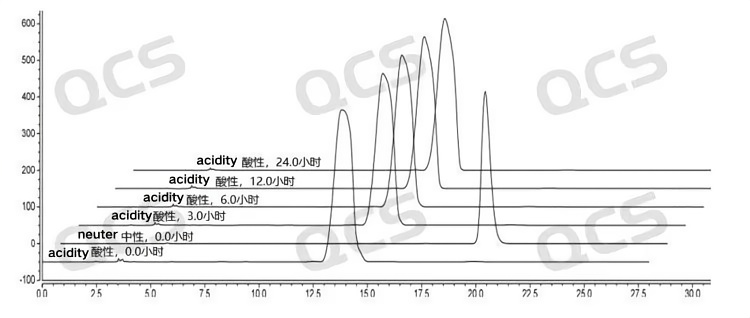
Figure 8 :Summary of Solution Stability Data for Sample RM-B192033 (F-97617-BM)
summary
In summary, through this experiment, we found that sample RM-B192001 (F-90021-RR) has good stability in both acidic and neutral solutions, but is unstable in alkaline solutions. Sample RM-B192001 (F-90021-RR) will undergo partial degradation during the preparation process with alkaline solution, and degradation will continue to occur with prolonged storage time. RM-B192014 (F-98226-BM) has good stability in acidic, neutral, and alkaline solutions. However, if the sample is dissolved in an alkaline diluent, it will cause a significant change in the retention time of the main peak of the sample. Therefore, customers should avoid using alkaline diluents when testing samples RM-B192001 (F-90021-RR) and RM-B192014 (F-98226-BM), and should not touch alkali during use, storage, and transportation. Sample RM-B192033 (F-97617-BM) has good stability in neutral and alkaline solutions, but is unstable in acidic solutions and will degrade completely during the preparation process with acidic solutions. Therefore, when testing sample RM-B192033 (F-97617-BM), customers should avoid using acidic diluents and should not touch acid during use, storage, and transportation. If customers have a need for the stability of these 3 samples, welcome to consult our company.


introduction
Following the qualitative study we published last year on "Sharing research on related impurities of Bilastine tablets for the Treatment of Rhinitis and Urticaria", today our center will continue to share the stability study of Bilastine specific impurities.
experimental scheme
In this experiment, our center conducted a study on the solubility stability of three specific impurities in the imported drug registration standard "Bilastine Tablets" (standard number JX20130329) issued by the State Food and Drug Administration, comparing the chromatographic conditions used under the "related substances" inspection item with three specific impurities in Bilastine. The sample item numbers and structural formulas used are shown in Figure 1 and Figure 2:

Figure 1: The impurity QCS item number and structure used in this study

Figure 2: Correspondence between Import Standard Impurity Code and QCS Item Number
In this experiment, The experimenter took appropriate amounts of RM-B192001(F-90021-RR; Bilastine Impurity A; CAS#2411093-91-7), RM-B192014(F-98226-BM; Bilastine Impurity 14; CAS#202189-83-1) and RM-B192033(F-97617-BM; Bilastine Impurity 33; CAS#2767509-00-0), then placed them in acidic, neutral and alkaline solutions, respectively, At room temperature and pressure for 0, 3, 6, 12, and 24 hours, follow the chromatographic conditions used under the "Related Substances" inspection item in the "Bilastine Tablets" Import Drug Registration Standard (Standard No. JX20130329) for injection detection. Observe the changes in the peak area of the main peak of the sample as the sample is placed for an extended period of time. Based on this, determine the stability of the sample solution
Empirical conclusion
RM-B192001(F-90021-RR)
After testing, it was found that the main peak area of sample RM-B192001 (F-90021-RR) did not change significantly after being placed in acidic and neutral solutions for 24 hours, with a relative standard deviation of less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and neutral solutions for 24 hours. However, during the process of placing the sample in alkaline solution for 24 hours, the area of the main peak continuously decreased. Comparing the spectra obtained by placing the sample in alkaline solution for 0.0 hours and in neutral solution for 0.0 hours, it can be found that the sample has already undergone partial degradation during the preparation of the sample solution with alkaline solution, and continued to degrade slowly during the process of placing it in alkaline solution from 0.0 hours to 24.0 hours The peak area data of the main peak at each detection point of sample RM-B192001 (F-90021-RR) under various pH conditions are as follows:

Figure 3: Summary of Solution Stability Data for Sample RM-B192001 (F-90021-RR)

Figure 4: Summary of solution stability data for sample RM-B192001 (F-90021-RR)
RM-B192014(F-98226-BM)
After testing, it was found that the main peak area of sample RM-B192014 (F-98226-BM) did not change significantly during 24 hours of storage in acidic and neutral solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and neutral solutions for 24 hours. However, the sample appears to be less stable in alkaline solution, and interesting "abnormal" experimental results were observed during alkaline stability testing of the sample.
When the sample was placed in an alkaline solution and immediately tested on the machine (at 0.0 hours in the figure), the obtained spectrum was compared with that obtained under neutral solution conditions. It was found that the retention time of the main peak of the spectrum obtained by the sample in alkaline solution changed significantly from that under neutral conditions, but the result did not further "deteriorate" with the extension of time. Moreover, after placing the sample in an alkaline solution for 24 hours and adjusting its pH to neutral, the retention time of the main peak of the sample was restored to be consistent with the results obtained under neutral solution conditions. The above results indicate that the "abnormal" results observed in sample RM-B192014 (F-98226-BM) under alkaline conditions are more likely to be due to changes in the ion state of the sample under this pH condition rather than sample degradation. The sample results can be restored with pH adjustment. Therefore, it can be considered that the sample is relatively stable when placed in an alkaline solution for 24 hours. The main peak peak area data of sample RM-B192014 (F-98226-BM) at each PH value is as follows:

Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-B192014 (F-98226-BM)

Figure 6: Summary of Solution Stability Data for Sample RM-B192014 (F-98226-BM)
RM-B192033(F-97617-BM)
After testing, it was found that the main peak area of sample RM-B192033 (F-97617-BM) did not change significantly during 24 hours of storage in alkaline and neutral solutions, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in neutral and alkaline solutions for 24 hours. However, the main peak area of the sample changed significantly after being placed in an acidic solution for 24 hours. Comparing the spectra obtained by placing the sample in an acidic solution for 0.0 hours with those obtained by placing it in a neutral solution for 0.0 hours, it can be found that the sample was completely degraded during the preparation of the sample solution with acidic solution. The peak area data of the main peak at each detection point of sample RM-B192033 (F-97617-BM) under various pH conditions are as follows:

Figure 7: Summary Line Chart of Solution Stability Data for Sample RM-B192033 (F-97617-BM)

Figure 8 :Summary of Solution Stability Data for Sample RM-B192033 (F-97617-BM)
summary
In summary, through this experiment, we found that sample RM-B192001 (F-90021-RR) has good stability in both acidic and neutral solutions, but is unstable in alkaline solutions. Sample RM-B192001 (F-90021-RR) will undergo partial degradation during the preparation process with alkaline solution, and degradation will continue to occur with prolonged storage time. RM-B192014 (F-98226-BM) has good stability in acidic, neutral, and alkaline solutions. However, if the sample is dissolved in an alkaline diluent, it will cause a significant change in the retention time of the main peak of the sample. Therefore, customers should avoid using alkaline diluents when testing samples RM-B192001 (F-90021-RR) and RM-B192014 (F-98226-BM), and should not touch alkali during use, storage, and transportation. Sample RM-B192033 (F-97617-BM) has good stability in neutral and alkaline solutions, but is unstable in acidic solutions and will degrade completely during the preparation process with acidic solutions. Therefore, when testing sample RM-B192033 (F-97617-BM), customers should avoid using acidic diluents and should not touch acid during use, storage, and transportation. If customers have a need for the stability of these 3 samples, welcome to consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号