Time:2024-08-02
After 14 months, EP (EDQM) finally acknowledged that impurity J in the current version of the European Pharmacopoeia was structurally incorrect and determined that impurity J in the new edition of the Pharmacopoeia will be defined as an unknown structure.
Cause of the event
In May 2023, our center received feedback from a customer that the Flucloxacillin Sodium EP Impurity J provided by us did not match the relative retention time (RRT) specified in the standard: the RRT for impurity J included in the pharmacopoeia standard was 1.57, but the customer's actual RRT was 0.96. According to the European Pharmacopoeia EP11.0, the structural formula of impurity J is shown in Figure 1
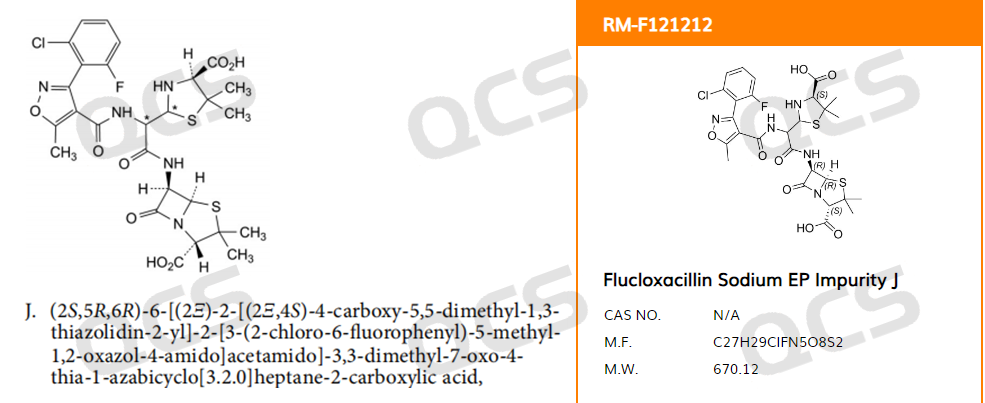
Figure 1: EP Flucloxacillin impurity J screenshot and QCS official website structure
Product verification
After receiving feedback from after-sales service, our center attached great importance to and organized efforts to conduct a detailed review of the relevant data of impurity J, and finally confirmed that our impurity J is structurally consistent with impurity J in the European Pharmacopoeia (EP). Based on our center's product data and customer feedback, we suspect that the information included in the EP Impurity J Pharmacopoeia in the current version may be incorrect. In order to verify our own inference, in addition to reviewing the structural confirmation spectrum, our company specifically purchased EP's Flucloxacillin for peak identification CRS, CAT: Y0002324) and conducted LC-MS data testing on the peak identification reference standard. After verification, no molecular weight signal related to EP impurity J (MW: 670.12) was detected after the main peak in the identification reference substance of EP's Flucloxacillin peak. The LC-MS data of the reference substance for the identification of Flucloxacillin peak are shown in Figure 2:
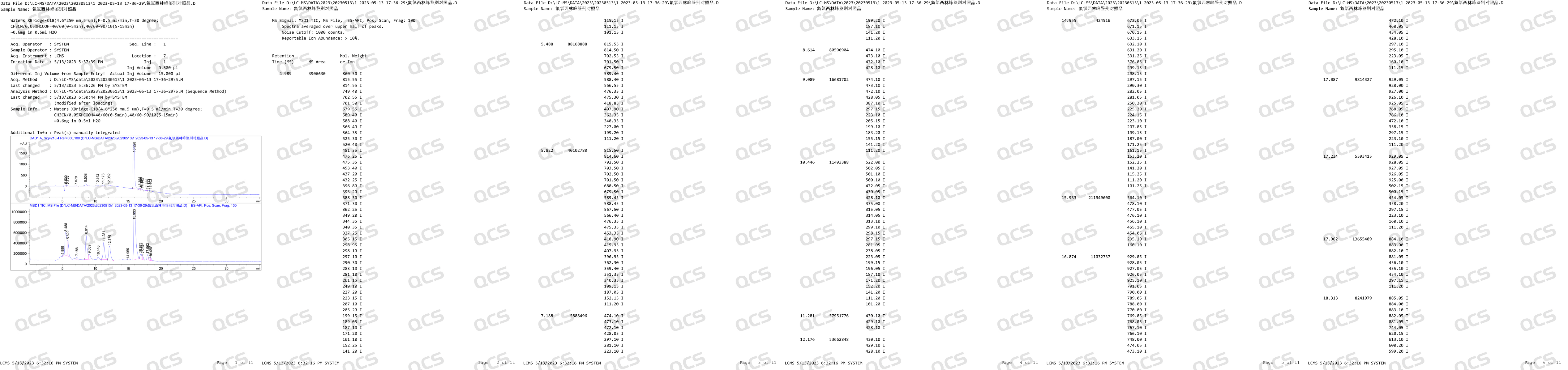
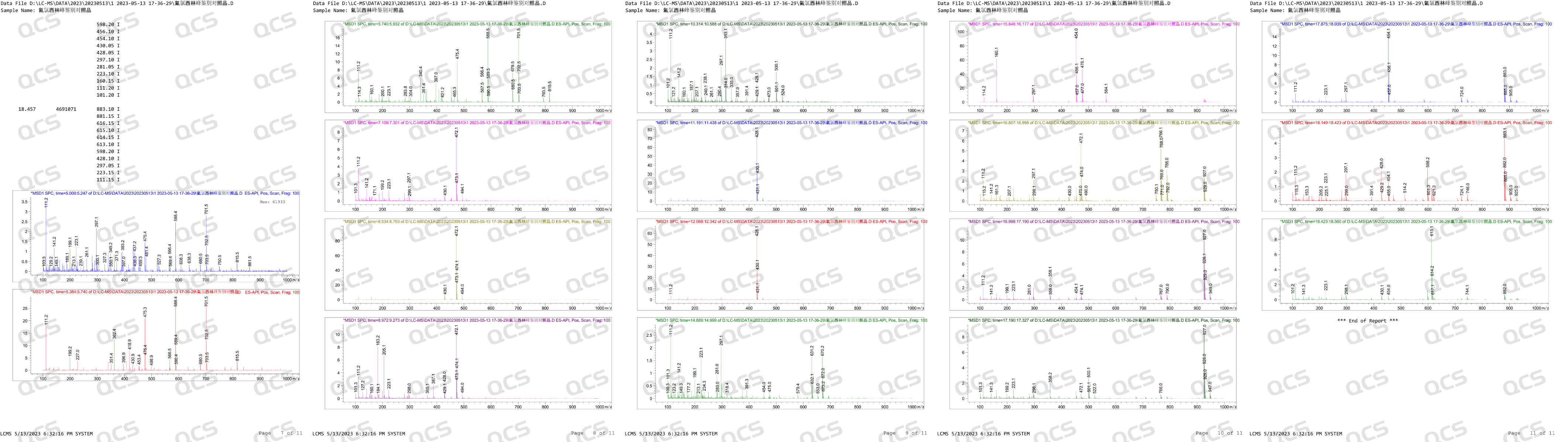
Figure 2: LC-MS data of EP Flucloxacillin peak identification reference substance
Based on the above test results and our product test data, we believe that the information included in the EP impurity J pharmacopoeia in the current version is indeed incorrect, the impurity J structure claimed in the current version of EP is not the component of the target peak with RRT of 1.5. Subsequently, our center shared the above information, as well as the structural identification data of impurity J and the testing data of CRS identification between impurity J and EP peaks, with the relevant personnel of EDQM (EP). At the same time, in October 2023, our center donated 20mg Flucloxacillin impurity J sample to EDQM for data validation, and the email content is shown in Figure 3 and Figure 4:

Figure 3: EP received feedback and replied to the email requesting a sample
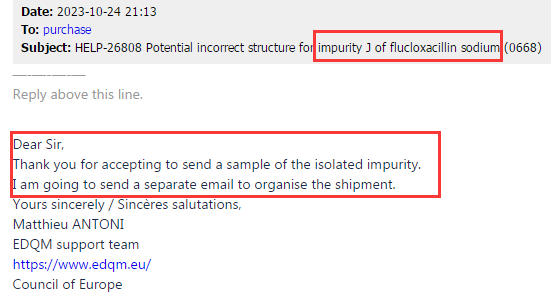
Figure 4: EP provides sample receiving information
Conclusion
Recently, while our center communicated with EDQM that the peaks of impurities B and G in Amikacin EP were inconsistent with the pharmacopoeia standard chromatogram, we also received a formal response from EP regarding Flucloxacillin impurity J.The reply fully affirmed our work and clearly stated that the structural formula of impurity J in the current version of EP is incorrect. This error will be corrected in the new version of EP, where impurity J will be presented as an unknown structure. This content will be reflected in EP11.8 version, and the new version is expected to take effect in July 2025. See Figure 5 for details:
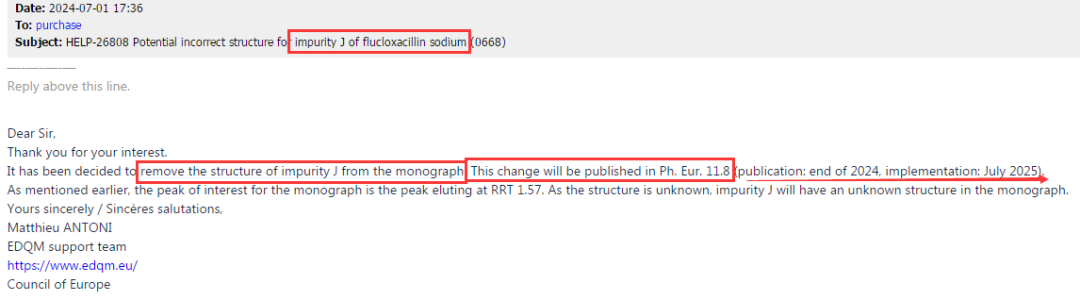
Figure 5: Reply from EP regarding Flucloxacillin impurity J and subsequent changes in various monograph
Summary
After 14 months, our center's data on the structure of Flucloxacillin EP impurity J was finally recognized by the European Pharmacopoeia Commission (EDQM) and acknowledged that the structure of Flucloxacillin EP impurity J was incorrect. EDQM has confirmed that the structural formula of impurity J will be removed from the 11.8 edition of the monograph.
Beyond topic
Scientific research is a serious matter that requires endurance, perseverance, and courage. We feel honored and gratified to be able to contribute to the development of the pharmaceutical industry. At the same time, we would like to express our gratitude to the customer for their continuous recognition and tolerance.


After 14 months, EP (EDQM) finally acknowledged that impurity J in the current version of the European Pharmacopoeia was structurally incorrect and determined that impurity J in the new edition of the Pharmacopoeia will be defined as an unknown structure.
Cause of the event
In May 2023, our center received feedback from a customer that the Flucloxacillin Sodium EP Impurity J provided by us did not match the relative retention time (RRT) specified in the standard: the RRT for impurity J included in the pharmacopoeia standard was 1.57, but the customer's actual RRT was 0.96. According to the European Pharmacopoeia EP11.0, the structural formula of impurity J is shown in Figure 1

Figure 1: EP Flucloxacillin impurity J screenshot and QCS official website structure
Product verification
After receiving feedback from after-sales service, our center attached great importance to and organized efforts to conduct a detailed review of the relevant data of impurity J, and finally confirmed that our impurity J is structurally consistent with impurity J in the European Pharmacopoeia (EP). Based on our center's product data and customer feedback, we suspect that the information included in the EP Impurity J Pharmacopoeia in the current version may be incorrect. In order to verify our own inference, in addition to reviewing the structural confirmation spectrum, our company specifically purchased EP's Flucloxacillin for peak identification CRS, CAT: Y0002324) and conducted LC-MS data testing on the peak identification reference standard. After verification, no molecular weight signal related to EP impurity J (MW: 670.12) was detected after the main peak in the identification reference substance of EP's Flucloxacillin peak. The LC-MS data of the reference substance for the identification of Flucloxacillin peak are shown in Figure 2:


Figure 2: LC-MS data of EP Flucloxacillin peak identification reference substance
Based on the above test results and our product test data, we believe that the information included in the EP impurity J pharmacopoeia in the current version is indeed incorrect, the impurity J structure claimed in the current version of EP is not the component of the target peak with RRT of 1.5. Subsequently, our center shared the above information, as well as the structural identification data of impurity J and the testing data of CRS identification between impurity J and EP peaks, with the relevant personnel of EDQM (EP). At the same time, in October 2023, our center donated 20mg Flucloxacillin impurity J sample to EDQM for data validation, and the email content is shown in Figure 3 and Figure 4:

Figure 3: EP received feedback and replied to the email requesting a sample

Figure 4: EP provides sample receiving information
Conclusion
Recently, while our center communicated with EDQM that the peaks of impurities B and G in Amikacin EP were inconsistent with the pharmacopoeia standard chromatogram, we also received a formal response from EP regarding Flucloxacillin impurity J.The reply fully affirmed our work and clearly stated that the structural formula of impurity J in the current version of EP is incorrect. This error will be corrected in the new version of EP, where impurity J will be presented as an unknown structure. This content will be reflected in EP11.8 version, and the new version is expected to take effect in July 2025. See Figure 5 for details:

Figure 5: Reply from EP regarding Flucloxacillin impurity J and subsequent changes in various monograph
Summary
After 14 months, our center's data on the structure of Flucloxacillin EP impurity J was finally recognized by the European Pharmacopoeia Commission (EDQM) and acknowledged that the structure of Flucloxacillin EP impurity J was incorrect. EDQM has confirmed that the structural formula of impurity J will be removed from the 11.8 edition of the monograph.
Beyond topic
Scientific research is a serious matter that requires endurance, perseverance, and courage. We feel honored and gratified to be able to contribute to the development of the pharmaceutical industry. At the same time, we would like to express our gratitude to the customer for their continuous recognition and tolerance.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号