Time:2024-07-30
Brief Introduction of Amikacin Sulfate EP Impurities
This article is a series. For the background, please refer to Issue 12, 2024 | Aminoglycoside antibiotics - Sharing on the stability study of Amikacin impurities and 24V13 | Uncovering the mystery of Amikacin unknown impurities. Welcome to participate in the benefits at the end of the article!
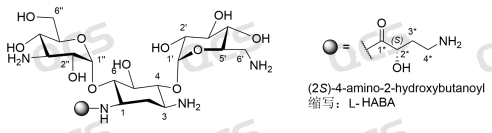
Figure 1: Amikacin impurity structure
Amikacin sulfate is a semisynthetic aminoglycoside antibiotic used primarily to treat serious infections caused by Gram-negative bacteria. Amikacin is chemically derived from kanamycin A, and its chemical name is 6-O-(3-Amino-3-deoxy-α-D-glucopyranosyl)-4-O-(6-amino-6-deoxy-α-D-glucopyranosyl)-1-N—[(2S)-4-amino-2-hydroxybutanoyl]-2-deoxy-D-streptamine sulfate
The synthesis process of Amikacin is as follows.
1. Starting material: Kanamycin A.
2. Chemical modification:
o N-acylation reaction: N-acylation reaction of the amino group of kanamycin A.
o L-HABA modification: selective acylation reaction of the exposed amino group at position 1 using L-(-)-γ-amino-α-hydroxybutyric acid (Chemical name: (2S)-4-amino-2-hydroxybutanoyl, abbreviation: L-HABA)
o Deacetylation reaction: selective deacetylation reaction is then carried out to obtain amikacin.
3. Sulfation: Finally, amikacin is converted to its sulfate form to improve its solubility and stability.
The chemical synthesis of amikacin involves the selectivity of functional groups and the interference of impurities in the starting reagents. Therefore, most of the impurities in amikacin sulfate listed in the European Pharmacopoeia (EP) are polyacylated impurities, acyl regioselective impurities or impurities introduced by the starting materials. (Scan the QR code at the end of the page to view all 24 impurity structures of amikacin)
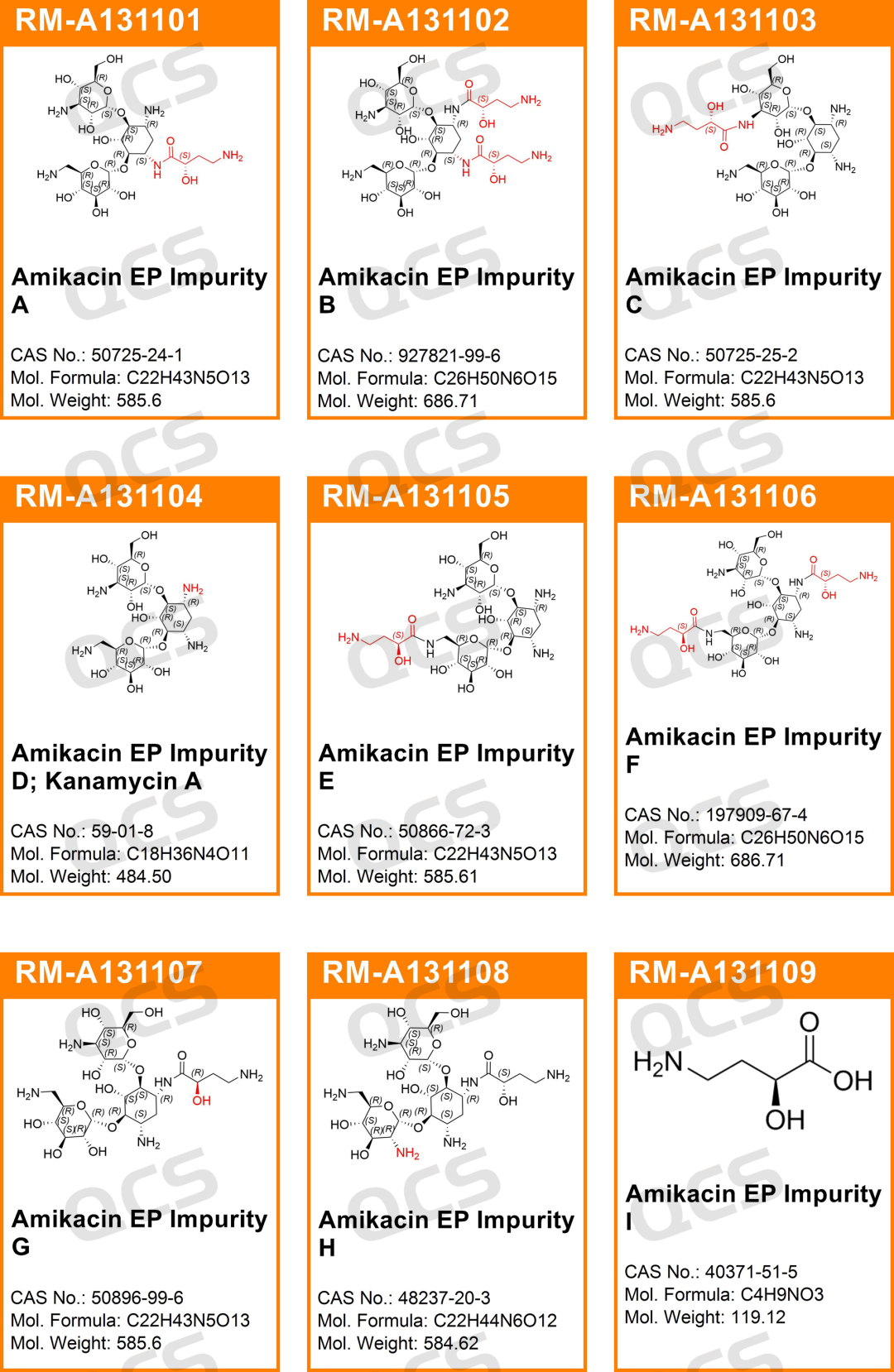
Figure 2: Impurities listed in the European Pharmacopoeia for Amikacin Sulfate
For example, impurity A is an impurity in which L-HABA is acylated at the 3-amino position. Impurity B is an impurity in which L-HABA is diacylated at the 1,3-amino positions. Impurity C is an impurity in which L-HABA is acylated at the 3’-amino position. Impurity D is the residual starting material Kanamycin A. Impurity E is an impurity in which L-HABA is acylated at the 6’-amino position. Impurity F is an impurity in which L-HABA is diacylated at the 1, 6’-amino positions. Impurity G is an impurity in which the isomer D-HABA is acylated at the 1-amino position. Impurity H is an impurity in which the 2’-hydroxyl group is converted to an amino group; and impurity I is the residual starting material L-HABA.
Below is the European Pharmacopoeia reference chromatogram.
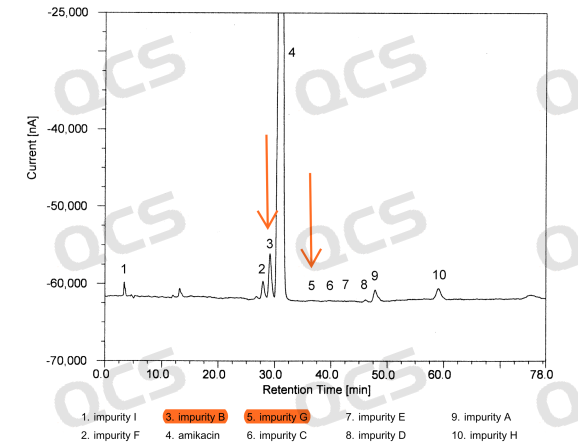
Figure 3: European Pharmacopoeia Reference Chromatogram
In order to verify the customer feedback results and solve the positioning problems in customer research, researchers in our center conducted directional synthesis of the two impurities respectively, and identified the synthesized standard samples through liquid chromatography, mass spectrometry, nuclear magnetic resonance hydrogen-carbon spectrum, and two-dimensional nuclear magnetic resonance techniques such as COSY, HSQC, HMBC, and NOESY, confirming that the structures of impurities B and G in our center are correct. Through comparative verification under liquid phase conditions, we believe that the European Pharmacopoeia has misplaced the attribution of amikacin impurities B and G, and the attribution of the two target peaks should be swapped.
The liquid chromatography results of amikacin sulfate, amikacin impurity B, and amikacin impurity G by the QCS Standard Material Research and Development Center are shown below.
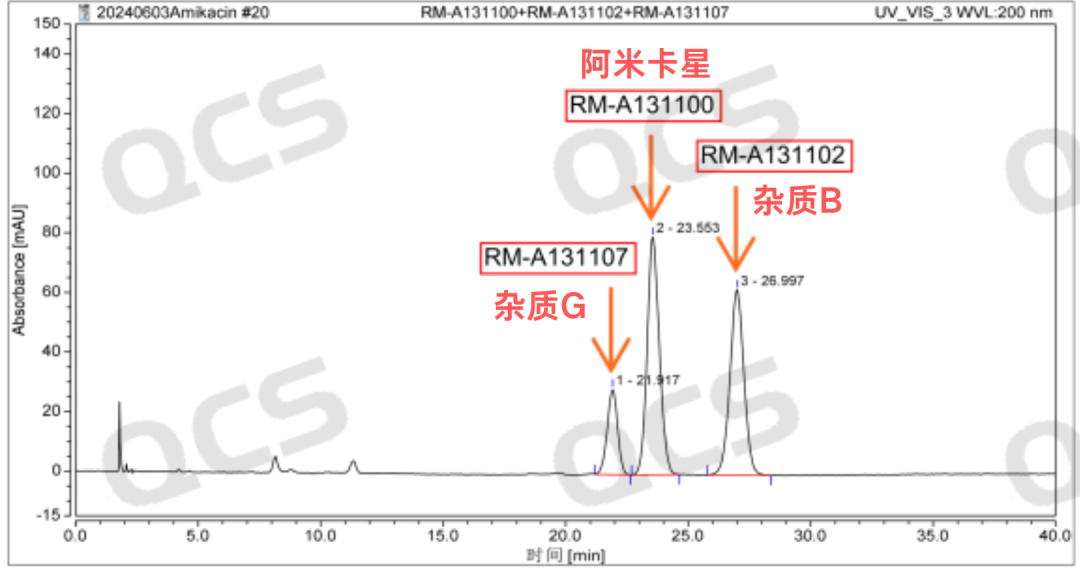
Figure 4: Testing results of impurities B and G in amikacin sulfate
The impurity standards used in this study have been fully characterized.
The characterization results of impurity B and impurity G are as follows.
Amikacin EP Impurity B (RM-A131102; CAS: 927821-99-6; Amikacin EP Impurity B) test data include HPLC, MS, HNMR, COSY, HSQC, HMBC, NOESY.
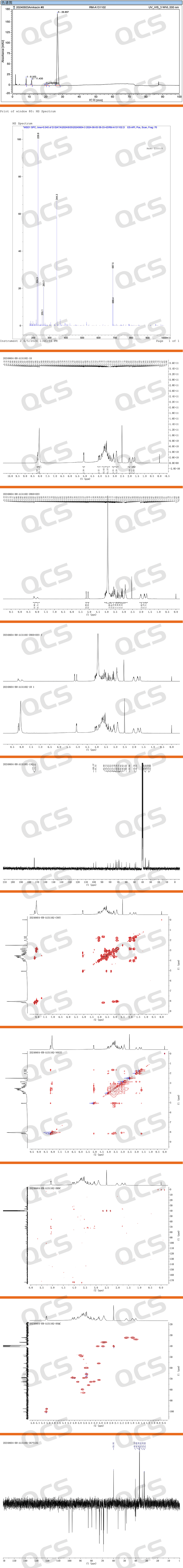
Figure 5: Test data of Amikacin Sulfate Impurity B
Amikacin EP Impurity G (RM-A131107; CAS: 50896-99-6; Amikacin EP Impurity G) test data includes HPLC, MS, HNMR, COSY, HSQC, HMBC, and NOESY.
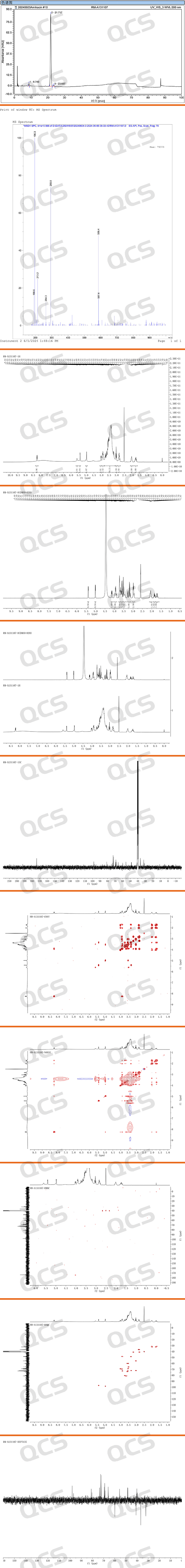
Figure 6: Test data of Amikacin Sulfate Impurity G
Summary
Since the impurities B and G of Amikacin EP have different molecular structures, the above two products can be well distinguished by mass spectrometry. At the same time, combined with hydrogen spectrum, carbon spectrum and two-dimensional nuclear magnetic resonance related evidence to prove that its structure is correct, the QCS Standard Material Research and Development Center used the identified standard samples to reproduce the liquid phase results recorded in the European Pharmacopoeia. Taking the amikacin sulfate raw material as a reference, it can be clearly observed in the liquid phase results that impurity G is eluted before the raw material, while impurity B is eluted later than the raw material. Therefore, we believe that the liquid chromatography peaks of impurities B and G included in the European Pharmacopoeia may need to be interchanged, and remind all relevant researchers to pay attention to the above issues. At the same time, in response to this issue, our center has simultaneously provided data feedback to the relevant departments of the European Pharmacopoeia (EDQM), and we will continue to update the subsequent progress.
Long press the identification QR code to view the list of all impurities!



Brief Introduction of Amikacin Sulfate EP Impurities
This article is a series. For the background, please refer to Issue 12, 2024 | Aminoglycoside antibiotics - Sharing on the stability study of Amikacin impurities and 24V13 | Uncovering the mystery of Amikacin unknown impurities. Welcome to participate in the benefits at the end of the article!

Figure 1: Amikacin impurity structure
Amikacin sulfate is a semisynthetic aminoglycoside antibiotic used primarily to treat serious infections caused by Gram-negative bacteria. Amikacin is chemically derived from kanamycin A, and its chemical name is 6-O-(3-Amino-3-deoxy-α-D-glucopyranosyl)-4-O-(6-amino-6-deoxy-α-D-glucopyranosyl)-1-N—[(2S)-4-amino-2-hydroxybutanoyl]-2-deoxy-D-streptamine sulfate
The synthesis process of Amikacin is as follows.
1. Starting material: Kanamycin A.
2. Chemical modification:
o N-acylation reaction: N-acylation reaction of the amino group of kanamycin A.
o L-HABA modification: selective acylation reaction of the exposed amino group at position 1 using L-(-)-γ-amino-α-hydroxybutyric acid (Chemical name: (2S)-4-amino-2-hydroxybutanoyl, abbreviation: L-HABA)
o Deacetylation reaction: selective deacetylation reaction is then carried out to obtain amikacin.
3. Sulfation: Finally, amikacin is converted to its sulfate form to improve its solubility and stability.
The chemical synthesis of amikacin involves the selectivity of functional groups and the interference of impurities in the starting reagents. Therefore, most of the impurities in amikacin sulfate listed in the European Pharmacopoeia (EP) are polyacylated impurities, acyl regioselective impurities or impurities introduced by the starting materials. (Scan the QR code at the end of the page to view all 24 impurity structures of amikacin)

Figure 2: Impurities listed in the European Pharmacopoeia for Amikacin Sulfate
For example, impurity A is an impurity in which L-HABA is acylated at the 3-amino position. Impurity B is an impurity in which L-HABA is diacylated at the 1,3-amino positions. Impurity C is an impurity in which L-HABA is acylated at the 3’-amino position. Impurity D is the residual starting material Kanamycin A. Impurity E is an impurity in which L-HABA is acylated at the 6’-amino position. Impurity F is an impurity in which L-HABA is diacylated at the 1, 6’-amino positions. Impurity G is an impurity in which the isomer D-HABA is acylated at the 1-amino position. Impurity H is an impurity in which the 2’-hydroxyl group is converted to an amino group; and impurity I is the residual starting material L-HABA.
Below is the European Pharmacopoeia reference chromatogram.

Figure 3: European Pharmacopoeia Reference Chromatogram
In order to verify the customer feedback results and solve the positioning problems in customer research, researchers in our center conducted directional synthesis of the two impurities respectively, and identified the synthesized standard samples through liquid chromatography, mass spectrometry, nuclear magnetic resonance hydrogen-carbon spectrum, and two-dimensional nuclear magnetic resonance techniques such as COSY, HSQC, HMBC, and NOESY, confirming that the structures of impurities B and G in our center are correct. Through comparative verification under liquid phase conditions, we believe that the European Pharmacopoeia has misplaced the attribution of amikacin impurities B and G, and the attribution of the two target peaks should be swapped.
The liquid chromatography results of amikacin sulfate, amikacin impurity B, and amikacin impurity G by the QCS Standard Material Research and Development Center are shown below.

Figure 4: Testing results of impurities B and G in amikacin sulfate
The impurity standards used in this study have been fully characterized.
The characterization results of impurity B and impurity G are as follows.
Amikacin EP Impurity B (RM-A131102; CAS: 927821-99-6; Amikacin EP Impurity B) test data include HPLC, MS, HNMR, COSY, HSQC, HMBC, NOESY.

Figure 5: Test data of Amikacin Sulfate Impurity B
Amikacin EP Impurity G (RM-A131107; CAS: 50896-99-6; Amikacin EP Impurity G) test data includes HPLC, MS, HNMR, COSY, HSQC, HMBC, and NOESY.

Figure 6: Test data of Amikacin Sulfate Impurity G
Summary
Since the impurities B and G of Amikacin EP have different molecular structures, the above two products can be well distinguished by mass spectrometry. At the same time, combined with hydrogen spectrum, carbon spectrum and two-dimensional nuclear magnetic resonance related evidence to prove that its structure is correct, the QCS Standard Material Research and Development Center used the identified standard samples to reproduce the liquid phase results recorded in the European Pharmacopoeia. Taking the amikacin sulfate raw material as a reference, it can be clearly observed in the liquid phase results that impurity G is eluted before the raw material, while impurity B is eluted later than the raw material. Therefore, we believe that the liquid chromatography peaks of impurities B and G included in the European Pharmacopoeia may need to be interchanged, and remind all relevant researchers to pay attention to the above issues. At the same time, in response to this issue, our center has simultaneously provided data feedback to the relevant departments of the European Pharmacopoeia (EDQM), and we will continue to update the subsequent progress.

Long press the identification QR code to view the list of all impurities!



Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号