Time:2024-07-26
Introduction
Today, we will share the original research impurity stability study of avatrombopag, a thrombocytopenia treatment drug. Avatrombopag is the world's first FDA-approved oral thrombopoietin receptor agonist (TPO-RA) for CLD-related thrombocytopenia, and is also the first small molecule innovative drug introduced by Fosun Pharma. As the first domestic treatment for chronic liver disease (CLD)-related thrombocytopenia, the launch of avatrombopag in my country fills the gap in the use of drugs in this field in China, and introduces a world-leading "strong, long-lasting, safe and convenient" new diagnosis and treatment plan for chronic liver disease (CLD)-related thrombocytopenia patients in China. It is mainly suitable for adult patients with chronic liver disease-related thrombocytopenia who undergo diagnostic procedures or surgery at an elective time.
Experimental Program
In this experiment, our center referred to the chromatographic conditions used under the "Related Substances" inspection item in "Avatrombopag Maleate Tablets" (standard number JX20190262) to study the solution stability of three original research code impurities of avatrombopag. The sample catalogue number and structure used are as shown in Figure 1 and the corresponding relationship between the impurity number and the original research code is shown in Figure 2.
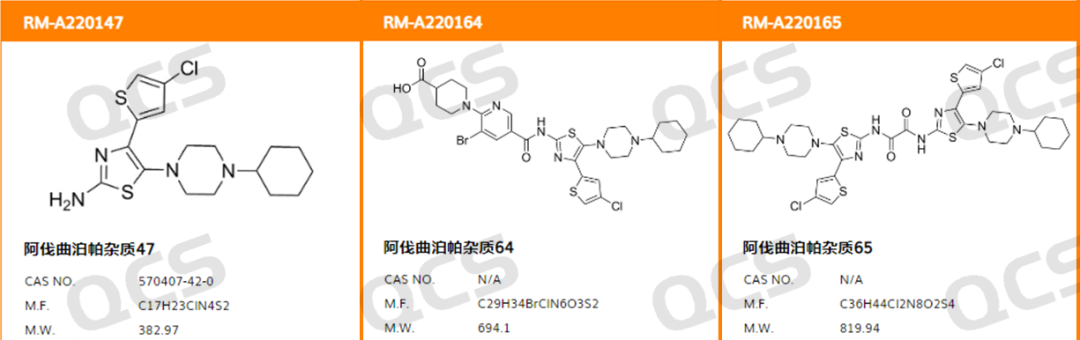
Figure 1: Impurity sample catalogue numbers and structures used in this study
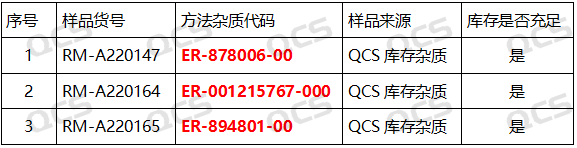
Figure 2: The corresponding relationship between the impurity code included in the standard and the impurity item number used in this study
In this experiment, the experimenter took appropriate amounts of RM-A220147 (ER-878006-00, Avatrombopag Impurity 47, CAS: 570407-42-0); RM-A220164 (ER-001215767-000, Avatrombopag Impurity 64) and RM-A220165 (ER-894801-00, Avatrombopag Impurity 65), placed them in acidic, neutral and alkaline solutions respectively, and placed them at room temperature and pressure for 0, 3, 6, 12 and 24 hours, respectively. Then, the samples were injected and tested according to the chromatographic conditions used under the "Related Substances" inspection item in "Avatrombopag Maleate Tablets" (standard number JX20190262). The change in the main peak area ratio (calculated by area normalization method) in the chromatogram was observed as the sample solution was placed for a longer period of time, and the solution stability of the sample was determined based on this.
Experimental Data and Conclusions
RM-A220147(ER-878006-00) and RM-A220164(ER-001215767)
After testing, we found that the main peak area percentage of samples RM-A220147 (ER-878006-00) and RM-A220164 (ER-001215767-000) did not change much during the 24-hour placement in acidic, neutral and alkaline solutions, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that samples RM-A220147 (ER-878006-00) and RM-A220164 (ER-001215767-000) are relatively stable during the 24-hour placement in acidic, neutral and alkaline solutions. The main peak area percentage data of each test point under various pH conditions are shown in Figures 3 and 4.
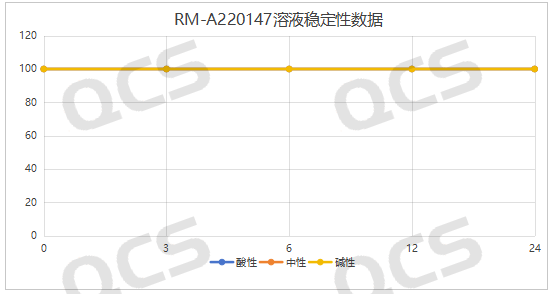
Figure 3: Summary of solution stability data for sample RM-A220147 (ER-878006-00)
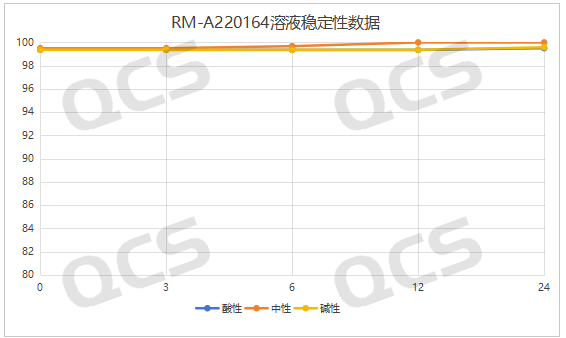
Figure 4: Summary of solution stability data of RM-A220164 (ER-001215767-000)
After testing, we found that sample RM-A220165 (ER-894801-00) is insoluble in neutral solution. The main peak area ratio does not change much during the 24-hour placement in acidic solution, and the relative standard deviation is less than 2.0%. However, the main peak area ratio of the sample changes greatly during the 24-hour placement in alkaline solution, and the relative standard deviation is greater than 2.0% (5.13%). Therefore, the sample is relatively stable during the 24-hour placement in acidic solution, but it is not stable during the 24-hour placement in alkaline solution, and it will slowly degrade. The data of the main peak area ratio of each test point of the sample under various pH conditions are shown in Figures 5-7.
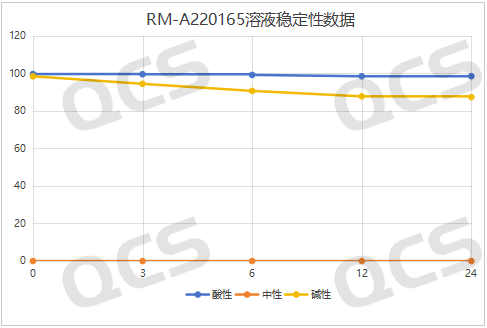
Figure 5: Comparison of acid solution stability data of RM-A220165 (ER-894801-000)
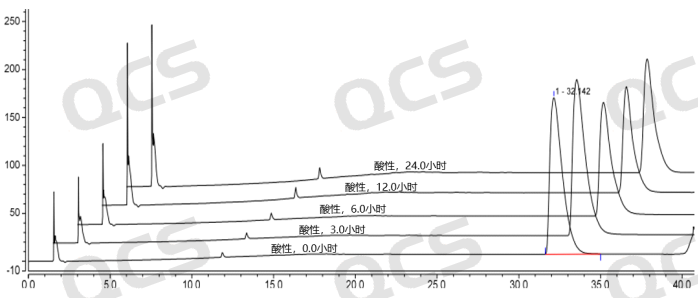
Figure 6: Comparison of neutral solution stability data of RM-A220165(ER-894801-000)
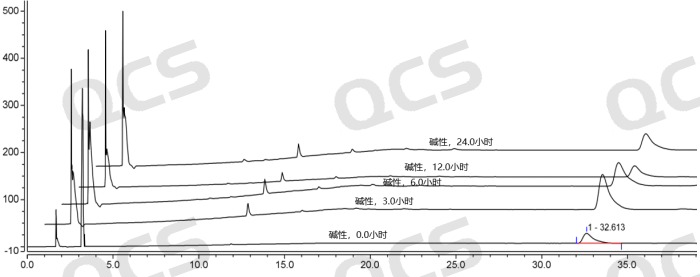
Figure 7: Comparison of alkaline solution stability data of RM-A220165 (ER-894801-000)
Summary
Through this experiment, we found that samples RM-A220147 (ER-878006-00) and RM-A220164 (ER-001215767-000) have good stability in acidic, alkaline and neutral solutions. Sample RM-A220165 (ER-894801-00) is insoluble in neutral solution (poor sample solubility), relatively stable in acidic solution, unstable in alkaline solution, and will slowly degrade if placed in alkaline solution for a long time. If customers need the stability content of these three samples, please consult our company.


Introduction
Today, we will share the original research impurity stability study of avatrombopag, a thrombocytopenia treatment drug. Avatrombopag is the world's first FDA-approved oral thrombopoietin receptor agonist (TPO-RA) for CLD-related thrombocytopenia, and is also the first small molecule innovative drug introduced by Fosun Pharma. As the first domestic treatment for chronic liver disease (CLD)-related thrombocytopenia, the launch of avatrombopag in my country fills the gap in the use of drugs in this field in China, and introduces a world-leading "strong, long-lasting, safe and convenient" new diagnosis and treatment plan for chronic liver disease (CLD)-related thrombocytopenia patients in China. It is mainly suitable for adult patients with chronic liver disease-related thrombocytopenia who undergo diagnostic procedures or surgery at an elective time.
Experimental Program
In this experiment, our center referred to the chromatographic conditions used under the "Related Substances" inspection item in "Avatrombopag Maleate Tablets" (standard number JX20190262) to study the solution stability of three original research code impurities of avatrombopag. The sample catalogue number and structure used are as shown in Figure 1 and the corresponding relationship between the impurity number and the original research code is shown in Figure 2.

Figure 1: Impurity sample catalogue numbers and structures used in this study

Figure 2: The corresponding relationship between the impurity code included in the standard and the impurity item number used in this study
In this experiment, the experimenter took appropriate amounts of RM-A220147 (ER-878006-00, Avatrombopag Impurity 47, CAS: 570407-42-0); RM-A220164 (ER-001215767-000, Avatrombopag Impurity 64) and RM-A220165 (ER-894801-00, Avatrombopag Impurity 65), placed them in acidic, neutral and alkaline solutions respectively, and placed them at room temperature and pressure for 0, 3, 6, 12 and 24 hours, respectively. Then, the samples were injected and tested according to the chromatographic conditions used under the "Related Substances" inspection item in "Avatrombopag Maleate Tablets" (standard number JX20190262). The change in the main peak area ratio (calculated by area normalization method) in the chromatogram was observed as the sample solution was placed for a longer period of time, and the solution stability of the sample was determined based on this.
Experimental Data and Conclusions
RM-A220147(ER-878006-00) and RM-A220164(ER-001215767)
After testing, we found that the main peak area percentage of samples RM-A220147 (ER-878006-00) and RM-A220164 (ER-001215767-000) did not change much during the 24-hour placement in acidic, neutral and alkaline solutions, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that samples RM-A220147 (ER-878006-00) and RM-A220164 (ER-001215767-000) are relatively stable during the 24-hour placement in acidic, neutral and alkaline solutions. The main peak area percentage data of each test point under various pH conditions are shown in Figures 3 and 4.

Figure 3: Summary of solution stability data for sample RM-A220147 (ER-878006-00)

Figure 4: Summary of solution stability data of RM-A220164 (ER-001215767-000)
After testing, we found that sample RM-A220165 (ER-894801-00) is insoluble in neutral solution. The main peak area ratio does not change much during the 24-hour placement in acidic solution, and the relative standard deviation is less than 2.0%. However, the main peak area ratio of the sample changes greatly during the 24-hour placement in alkaline solution, and the relative standard deviation is greater than 2.0% (5.13%). Therefore, the sample is relatively stable during the 24-hour placement in acidic solution, but it is not stable during the 24-hour placement in alkaline solution, and it will slowly degrade. The data of the main peak area ratio of each test point of the sample under various pH conditions are shown in Figures 5-7.

Figure 5: Comparison of acid solution stability data of RM-A220165 (ER-894801-000)

Figure 6: Comparison of neutral solution stability data of RM-A220165(ER-894801-000)

Figure 7: Comparison of alkaline solution stability data of RM-A220165 (ER-894801-000)
Summary
Through this experiment, we found that samples RM-A220147 (ER-878006-00) and RM-A220164 (ER-001215767-000) have good stability in acidic, alkaline and neutral solutions. Sample RM-A220165 (ER-894801-00) is insoluble in neutral solution (poor sample solubility), relatively stable in acidic solution, unstable in alkaline solution, and will slowly degrade if placed in alkaline solution for a long time. If customers need the stability content of these three samples, please consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号