Time:2024-07-20
Introduction
Following the last article, "Aminoglycoside antibiotics-Amikacin impurity stability research sharing", today we will share the discovery and development process of four unknown degradable impurities of Amikacin.
Discovery
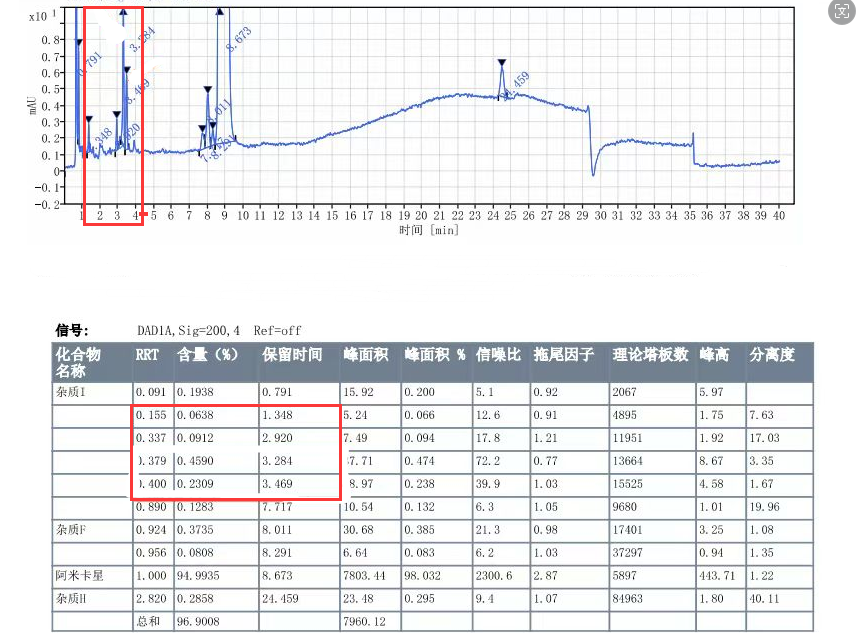
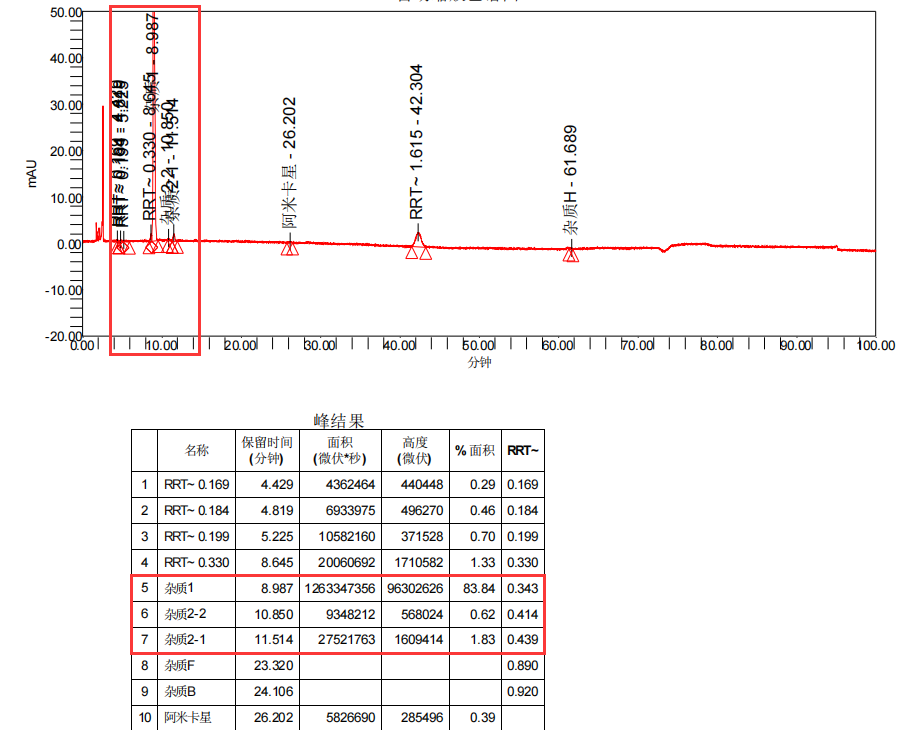
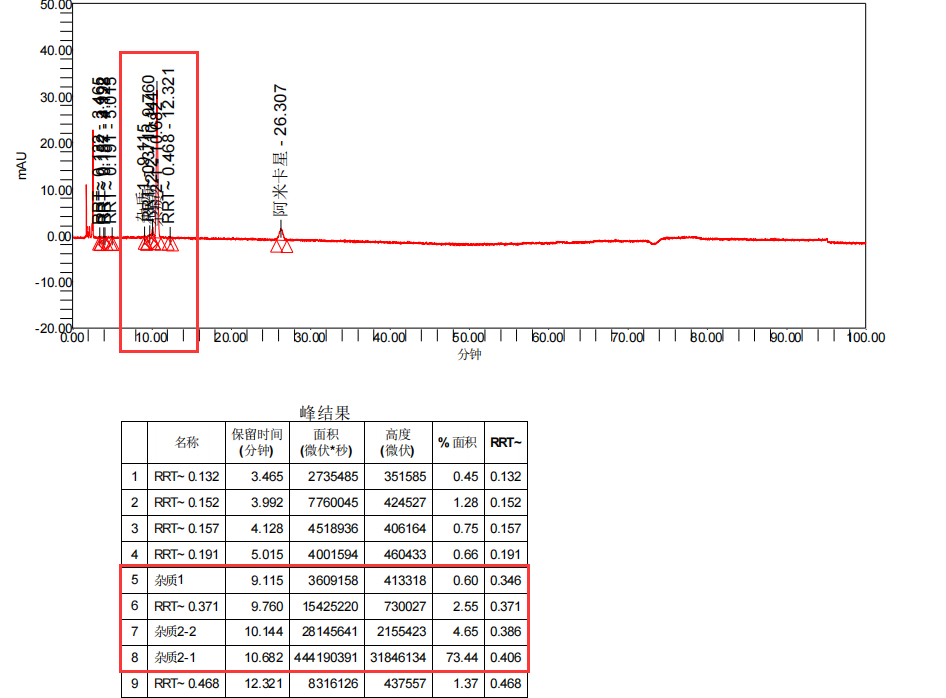
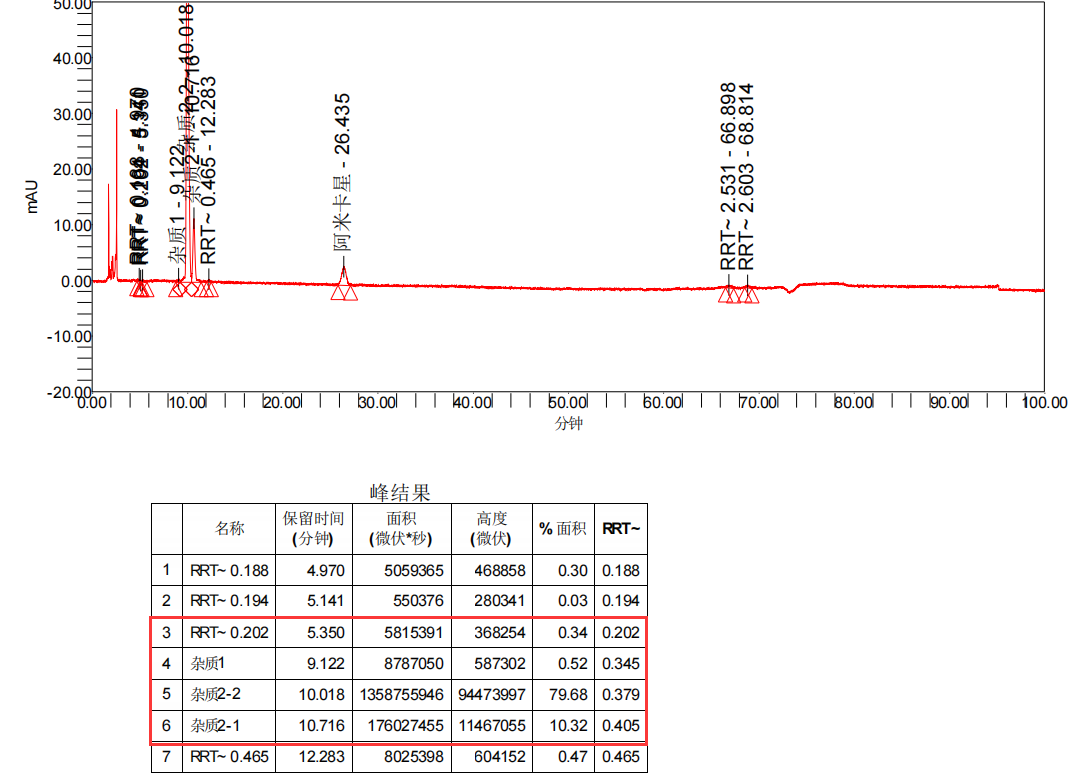
Figure 1: Customer's rough product drawing (four drawings)
Amikacin (sulfate) is an aminoglycoside antibiotic, which can be considered a relatively old variety. However, based on current market feedback, there are many manufacturers producing. Recently, we have received feedback from multiple customers that their Amikacin products have produced four unknown large impurities during long-term stability studies. These impurities have a retention time mainly concentrated between 4-10 minutes, calculated as relative retention time (RRT): 0.18~0.2 (unknown impurity 1), 0.35~0.36 (unknown impurity 2), 0.37~0.38 (unknown impurity 3), and 0.4~0.42 (unknown impurity 4) intervals, respectively. The detailed impurity information data are summarized in Figure 2:
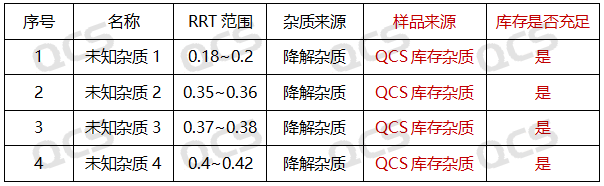
Figure 2: Summary of relevant information data for four unknown impurities
Based on the existing information, it can be determined that the above impurities are not known structural impurities included in the pharmacopoeia. However, by searching relevant literature and pharmacopoeia data, we found that the above impurity signals have corresponding signals appearing in the typical chromatogram or system applicability spectrum of the European Pharmacopoeia (EP). However, this impurity signal has not been clearly identified by the European Pharmacopoeia, as shown in Figure 3:

Figure 3: European Pharmacopoeia chromatogram information (two figures)
Preparation and purification of the impurities
For the research needs of impurities that are not fully characterized in the European Pharmacopoeia but appear in actual samples, our center has carried out special development. In view of the customers' demand for the study of the unknown impurities of Amikacin, our center first relied on the preparation and separation department to carry out preparation and purification work on rough products containing target impurities.
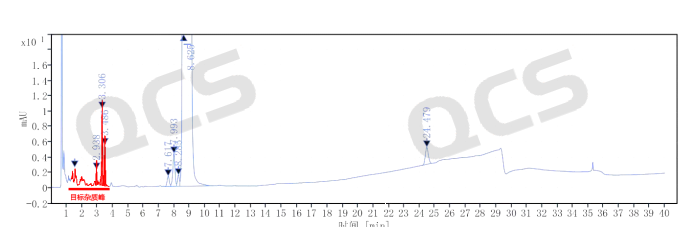
From the rough product map, it can be seen that the retention time of the four unknown impurities is small and the difference in peak time is small. At the same time, under these conditions, there are many salts in the detection conditions of Amikacin that will have a significant impact on the preparation of impurities. The QCS Standard Material R&D Center has invested over four months in the development and optimization of preparation methods in the preparation and separation laboratory, ultimately achieving good separation of target impurities. During the entire development and production process, the following four major challenges are mainly faced:
First: The polarity of Amikacin is too high, and the retention time of the four target impurities is too small, requiring separate development of analysis and preparation methods.
Second:The four target impurities are too close together, and only separation conditions close to the Amikacin standard analysis method(100min/needle) can effectively separate these four impurities. Therefore, the preparation process faces great technical challenges, and the final optimized preparation method requires a time of 120min/needle
Third: Due to the poor separation of the target impurity peak, the single injection volume is very small, less than 20mg/injection. The enrichment cycle of the product far exceeds our expectations (120min/injection, only 4 injections can be injected in 8 hours a day, and the daily rough product injection volume is less than 100mg). At the same time, it is difficult to purify the rough product completely at once, often requiring multiple preparations.
Fourth: Due to the use of a large amount of salt in product analysis methods, the purified product solution contains salt, making desalination a very challenging task.
Research on impurities
Finally, through the continuous efforts and hard work of our preparation colleagues, we overcame the various difficulties mentioned above and finally prepared and separated four unknown degradation impurity samples. The analysis colleagues identified four impurities according to the pharmacopoeia method, and completed separate 1:1 mixing of Amikacin API and four unknown impurities, as well as overall mixing of four unknown impurities and API in bulk. Now share the total mixed injection data of Amikacin API+unknown impurity 1+unknown impurity 2+unknown impurity 3+unknown impurity 4, as shown in Figure 4:
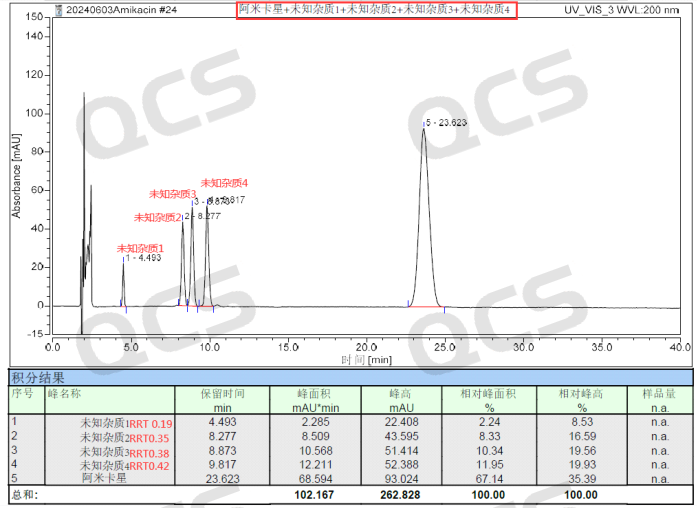
Figure 4: Mixed injection data of four unknown impurities and Amikacin
Finally, our center has basically completed the relevant research on the degradation of impurities in Amikacin. The above unknown impurities can now be provided with related products. Interested customers are welcome to come and inquire and communicate with us.
Share and obtain dry goods benefits
Although Amikacin is a very old variety and there are many customers studying it, most customers in the market are currently providing feedback that the detection time of Amikacin impurity B and impurity G under standard conditions is reversed. Impurity B is located behind the main peak, while impurity G is located before it, which contradicts the typical chromatogram or system suitability spectrum of EP. Many customers question the correctness of the product, but no official corrections or other materials have been retrieved. In the next issue, we will focus on sharing the relevant research on Amikacin EP impurity B/G. We welcome everyone to actively participate in the discussion.
Forward this article and add the text “Amikacin EP impurity B/G peak does not match the standard, how will we deal with it?" to have the opportunity to obtain two-dimensional nuclear magnetic resonance data of Amikacin EP impurity B/G
Long press to recognize the QR code and participate in this event!



Introduction
Following the last article, "Aminoglycoside antibiotics-Amikacin impurity stability research sharing", today we will share the discovery and development process of four unknown degradable impurities of Amikacin.
Discovery




Figure 1: Customer's rough product drawing (four drawings)
Amikacin (sulfate) is an aminoglycoside antibiotic, which can be considered a relatively old variety. However, based on current market feedback, there are many manufacturers producing. Recently, we have received feedback from multiple customers that their Amikacin products have produced four unknown large impurities during long-term stability studies. These impurities have a retention time mainly concentrated between 4-10 minutes, calculated as relative retention time (RRT): 0.18~0.2 (unknown impurity 1), 0.35~0.36 (unknown impurity 2), 0.37~0.38 (unknown impurity 3), and 0.4~0.42 (unknown impurity 4) intervals, respectively. The detailed impurity information data are summarized in Figure 2:

Figure 2: Summary of relevant information data for four unknown impurities
Based on the existing information, it can be determined that the above impurities are not known structural impurities included in the pharmacopoeia. However, by searching relevant literature and pharmacopoeia data, we found that the above impurity signals have corresponding signals appearing in the typical chromatogram or system applicability spectrum of the European Pharmacopoeia (EP). However, this impurity signal has not been clearly identified by the European Pharmacopoeia, as shown in Figure 3:

Figure 3: European Pharmacopoeia chromatogram information (two figures)
Preparation and purification of the impurities
For the research needs of impurities that are not fully characterized in the European Pharmacopoeia but appear in actual samples, our center has carried out special development. In view of the customers' demand for the study of the unknown impurities of Amikacin, our center first relied on the preparation and separation department to carry out preparation and purification work on rough products containing target impurities.

From the rough product map, it can be seen that the retention time of the four unknown impurities is small and the difference in peak time is small. At the same time, under these conditions, there are many salts in the detection conditions of Amikacin that will have a significant impact on the preparation of impurities. The QCS Standard Material R&D Center has invested over four months in the development and optimization of preparation methods in the preparation and separation laboratory, ultimately achieving good separation of target impurities. During the entire development and production process, the following four major challenges are mainly faced:
First: The polarity of Amikacin is too high, and the retention time of the four target impurities is too small, requiring separate development of analysis and preparation methods.
Second:The four target impurities are too close together, and only separation conditions close to the Amikacin standard analysis method(100min/needle) can effectively separate these four impurities. Therefore, the preparation process faces great technical challenges, and the final optimized preparation method requires a time of 120min/needle
Third: Due to the poor separation of the target impurity peak, the single injection volume is very small, less than 20mg/injection. The enrichment cycle of the product far exceeds our expectations (120min/injection, only 4 injections can be injected in 8 hours a day, and the daily rough product injection volume is less than 100mg). At the same time, it is difficult to purify the rough product completely at once, often requiring multiple preparations.
Fourth: Due to the use of a large amount of salt in product analysis methods, the purified product solution contains salt, making desalination a very challenging task.
Research on impurities
Finally, through the continuous efforts and hard work of our preparation colleagues, we overcame the various difficulties mentioned above and finally prepared and separated four unknown degradation impurity samples. The analysis colleagues identified four impurities according to the pharmacopoeia method, and completed separate 1:1 mixing of Amikacin API and four unknown impurities, as well as overall mixing of four unknown impurities and API in bulk. Now share the total mixed injection data of Amikacin API+unknown impurity 1+unknown impurity 2+unknown impurity 3+unknown impurity 4, as shown in Figure 4:

Figure 4: Mixed injection data of four unknown impurities and Amikacin
Finally, our center has basically completed the relevant research on the degradation of impurities in Amikacin. The above unknown impurities can now be provided with related products. Interested customers are welcome to come and inquire and communicate with us.
Share and obtain dry goods benefits
Although Amikacin is a very old variety and there are many customers studying it, most customers in the market are currently providing feedback that the detection time of Amikacin impurity B and impurity G under standard conditions is reversed. Impurity B is located behind the main peak, while impurity G is located before it, which contradicts the typical chromatogram or system suitability spectrum of EP. Many customers question the correctness of the product, but no official corrections or other materials have been retrieved. In the next issue, we will focus on sharing the relevant research on Amikacin EP impurity B/G. We welcome everyone to actively participate in the discussion.
Forward this article and add the text “Amikacin EP impurity B/G peak does not match the standard, how will we deal with it?" to have the opportunity to obtain two-dimensional nuclear magnetic resonance data of Amikacin EP impurity B/G
Long press to recognize the QR code and participate in this event!



Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号