Time:2024-07-14
Introduction
Today we will share the research Aminoglycoside antibiotics - Amikacin related impurities, Amikacin is an aminoglycoside antibiotic that can act on the ribosomes in bacteria, inhibit protein synthesis, and disrupt the integrity of bacterial cell walls, leading to membrane damage and cell death. Applicable to infections caused by Gram negative bacteria and penicillin resistant Staphylococcus aureus. Intramuscular injection or intravenous drip administration, with a half-life of approximately 2 hours for intravenous drip, and can last up to 30 hours without urine, with little protein binding. Mainly causing damage to the cochlear nerve, ototoxicity and nephrotoxicity are similar to those of gentamicin. Amikacin exerts its antibacterial effect by binding to the bacterial 30S subunit, blocking bacterial protein synthesis.
Experimental scheme
Experimental preparation
In this experiment, our center referred to the chromatographic conditions used in the "Related Substances" inspection under the "Amikacin" variety item in Part II of the Chinese Pharmacopoeia (2020 edition) to monitor the inclusion in the pharmacopoeia, conducted liquid chromatography localization studies on two impurities in stock to check if the system's applicability meets the requirements of the pharmacopoeia. And the solution stability of three code impurities in Amikacin was studied. Sample item number, code and structure formula used are as shown in Figure 1 and Figure 2:
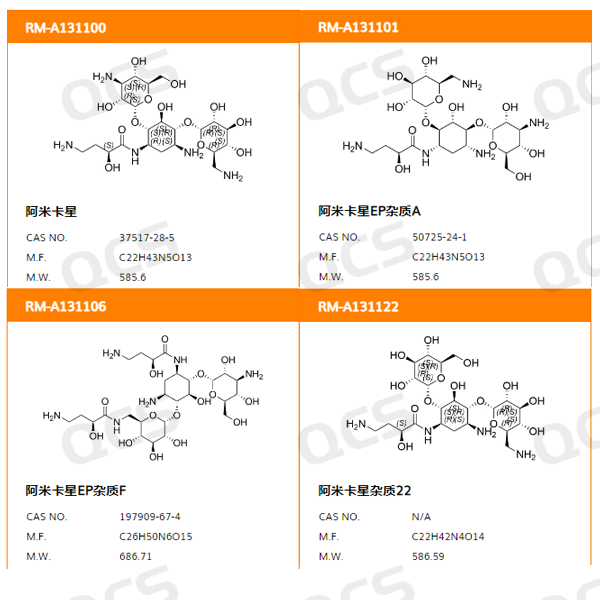
Figure 1: Impurity item number and structural formula
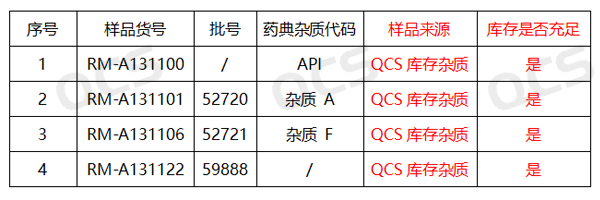
Figure 2: Corresponding diagram between impurity item number and code
Experimental Plan for the Applicability of Amikacin System
In this experiment, the experimenter refers to the chromatographic conditions used in the "Related Substances" inspection under the "Amikacin" variety item in Part II of the Chinese Pharmacopoeia (2020 edition), take RM-A131100 (API; Amikacin, CAS: 37517-28-5), RM-A131101 (Impurity A; Amikacin EP Impurity A, CAS: 50725-24-1) and RM-A131106 (impurity F, Amikacin EP Impurity F, CAS: 197909-67-4) were prepared as sample solutions, and injected separately, then ,the three sample solutions were mixed and injected again. Calculate the RRT of Pharmacopoeia Impurity A and Pharmacopoeia Impurity F based on the detected chromatogram, and determine whether the system suitability meets the pharmacopoeia regulations by comparing the calculated RRT value with the pharmacopoeia specified RRT value through comparative experiments.
A & F & RRT 0.32 study plan for Amikacin impurity
In this experiment, the experimenter took appropriate amounts of samples RM-A131101 (impurity A), RM-A131106 (impurity F) and impurity RM-A131122 (RRT 0.32) and placed them in acidic, neutral and alkaline solutions, respectively. They were placed at room temperature and pressure for 0,3,6,12 and 24 hours, and then injected for detection according to the chromatographic conditions used in the "related substances" inspection under the “Amikacin”variety item in part 2 of the "Chinese Pharmacopoeia" (2020 edition). Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Experiment conclusion
Experimental conclusion of Amikacin system applicability
Although the stationary phase of the chromatographic column in this study was not exactly the same as the chromatographic column specified in the Pharmacopoeia, the two codes of impurity A and impurity F included in the "Related Substances" inspection item under the "Amikacin" variety item in Part II of the Chinese Pharmacopoeia (2020 edition) were used as the basis for determination. The measured relative retention time was basically consistent with the data in the standard, so the adaptability of this experimental system is good. The test chromatogram is shown in Figure 3, and the comparison data between the calculated RRT value and the RRT value specified in the pharmacopoeia are shown in Figure 4:
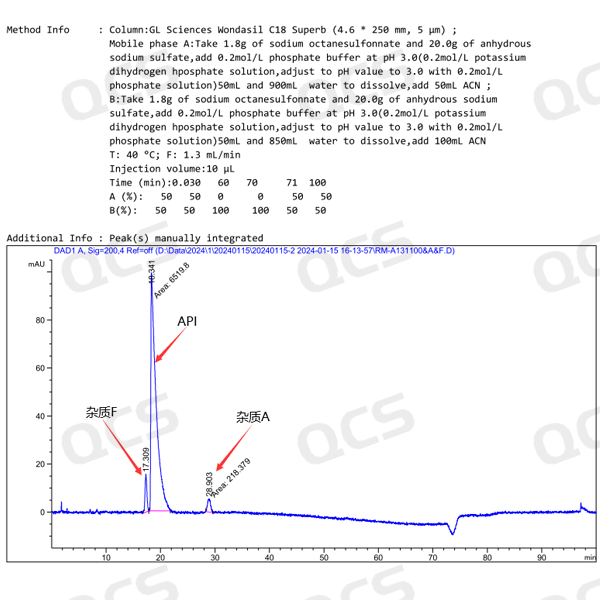
Figure 3: Mixed injection chromatogram of impurity A and impurity F included in the Chinese Pharmacopoeia (2020 edition) Part II "Amikacin"
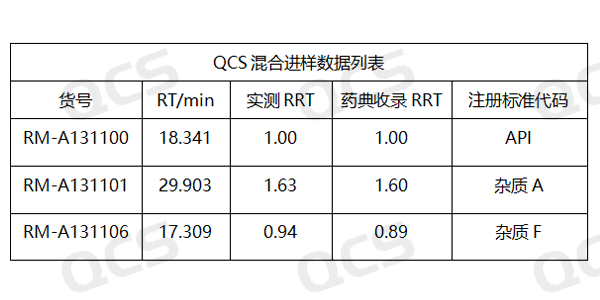
Figure 4: Comparison data between RRT values obtained from experimental calculations and RRT values specified in the pharmacopoeia
After testing, it was found that the main peak area of sample RM-A131122 (RRT 0.32) did not change significantly during 24 hours of storage in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that this sample is relatively stable in the acidic, neutral and alkaline solution for 24 hours. The test data of this sample in different pH solutions are summarized as follows:
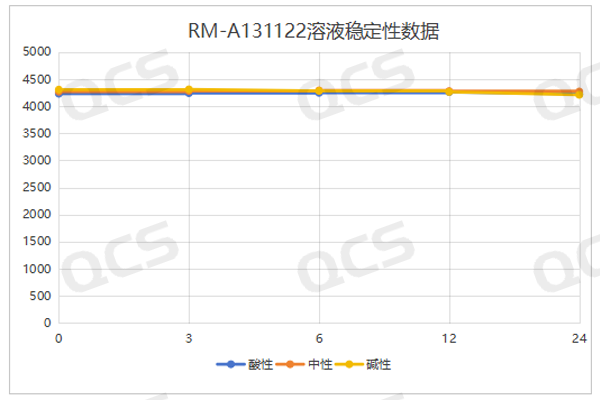
Figure 5: RM-A131122, Summary of Solution Stability Data
Blue color:acidic; Orange color: neutral ; Yellow color:alkaline
When the samples RM-A131101 (impurity A) and RM-A131106 (impurity F) were placed in acidic and neutral solutions for 24 hours, the main peak area did not change significantly, and the relative standard deviation was less than 2.0%. However, during the process of samples in alkaline solution for 24 hours, the main peak area changed greatly, and the relative standard deviation was greater than 2.0% (the relative standard deviation of impurity A was 2.73%, and the relative standard deviation of impurity F was 4.59%), and the impurity increased significantly. So these two samples are relatively stable in acidic and neutral solutions, and will undergo slow degradation in alkaline solutions. The test data of these 2 samples in solution environments with different pH values are summarized as follows:
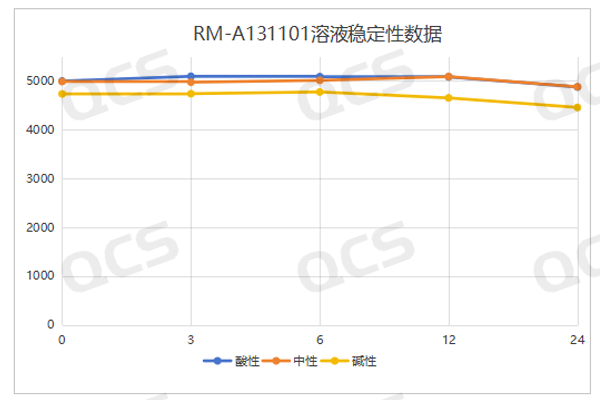
Figure 6: RM-A131101 Summary of Solution Stability Data
Blue color:acidic; Orange color: neutral ; Yellow color:alkaline
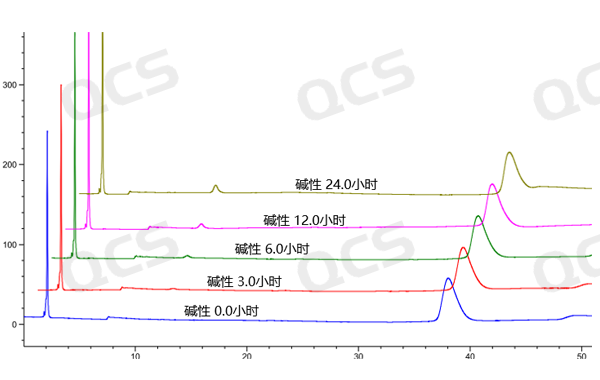
Figure 7: RM-A131101 3D plot of solution stability data
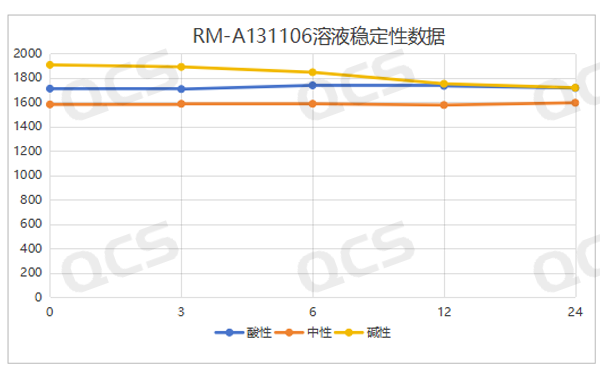
Figure 8: RM-A131106 Summary of Solution Stability Data
Blue color:acidic; Orange color: neutral ; Yellow color:alkaline
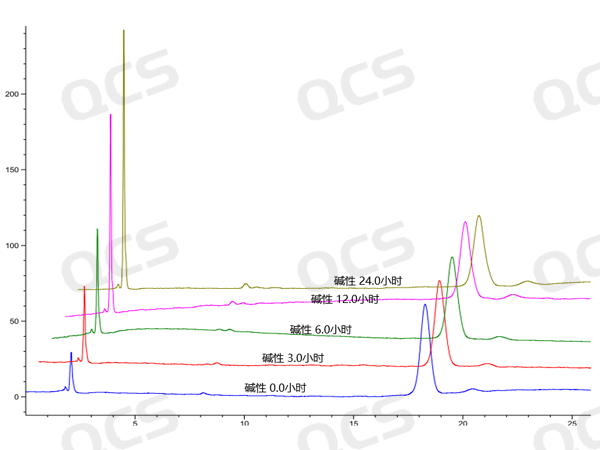
Figure 9:RM-A131106 3D plot of solution stability data
Summary
In summary, through this experiment, we found that sample RM-A131122 (RRT 0.32) has good stability in acidic, neutral, and alkaline solutions. Samples RM-A131101 (Impurity A) and RM-A131106 (Impurity F) have good stability in acidic and neutral solutions, but poor stability in alkaline solutions, they will undergo slow degradation with prolonged storage time, attention should be paid during storage and testing, and it is recommended to prepare and use them as needed, they are not suitable as reserve solutions. If the stability data of the above 3 samples is needed, welcome to consult our company.


Introduction
Today we will share the research Aminoglycoside antibiotics - Amikacin related impurities, Amikacin is an aminoglycoside antibiotic that can act on the ribosomes in bacteria, inhibit protein synthesis, and disrupt the integrity of bacterial cell walls, leading to membrane damage and cell death. Applicable to infections caused by Gram negative bacteria and penicillin resistant Staphylococcus aureus. Intramuscular injection or intravenous drip administration, with a half-life of approximately 2 hours for intravenous drip, and can last up to 30 hours without urine, with little protein binding. Mainly causing damage to the cochlear nerve, ototoxicity and nephrotoxicity are similar to those of gentamicin. Amikacin exerts its antibacterial effect by binding to the bacterial 30S subunit, blocking bacterial protein synthesis.
Experimental scheme
Experimental preparation
In this experiment, our center referred to the chromatographic conditions used in the "Related Substances" inspection under the "Amikacin" variety item in Part II of the Chinese Pharmacopoeia (2020 edition) to monitor the inclusion in the pharmacopoeia, conducted liquid chromatography localization studies on two impurities in stock to check if the system's applicability meets the requirements of the pharmacopoeia. And the solution stability of three code impurities in Amikacin was studied. Sample item number, code and structure formula used are as shown in Figure 1 and Figure 2:

Figure 1: Impurity item number and structural formula

Figure 2: Corresponding diagram between impurity item number and code
Experimental Plan for the Applicability of Amikacin System
In this experiment, the experimenter refers to the chromatographic conditions used in the "Related Substances" inspection under the "Amikacin" variety item in Part II of the Chinese Pharmacopoeia (2020 edition), take RM-A131100 (API; Amikacin, CAS: 37517-28-5), RM-A131101 (Impurity A; Amikacin EP Impurity A, CAS: 50725-24-1) and RM-A131106 (impurity F, Amikacin EP Impurity F, CAS: 197909-67-4) were prepared as sample solutions, and injected separately, then ,the three sample solutions were mixed and injected again. Calculate the RRT of Pharmacopoeia Impurity A and Pharmacopoeia Impurity F based on the detected chromatogram, and determine whether the system suitability meets the pharmacopoeia regulations by comparing the calculated RRT value with the pharmacopoeia specified RRT value through comparative experiments.
A & F & RRT 0.32 study plan for Amikacin impurity
In this experiment, the experimenter took appropriate amounts of samples RM-A131101 (impurity A), RM-A131106 (impurity F) and impurity RM-A131122 (RRT 0.32) and placed them in acidic, neutral and alkaline solutions, respectively. They were placed at room temperature and pressure for 0,3,6,12 and 24 hours, and then injected for detection according to the chromatographic conditions used in the "related substances" inspection under the “Amikacin”variety item in part 2 of the "Chinese Pharmacopoeia" (2020 edition). Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Experiment conclusion
Experimental conclusion of Amikacin system applicability
Although the stationary phase of the chromatographic column in this study was not exactly the same as the chromatographic column specified in the Pharmacopoeia, the two codes of impurity A and impurity F included in the "Related Substances" inspection item under the "Amikacin" variety item in Part II of the Chinese Pharmacopoeia (2020 edition) were used as the basis for determination. The measured relative retention time was basically consistent with the data in the standard, so the adaptability of this experimental system is good. The test chromatogram is shown in Figure 3, and the comparison data between the calculated RRT value and the RRT value specified in the pharmacopoeia are shown in Figure 4:

Figure 3: Mixed injection chromatogram of impurity A and impurity F included in the Chinese Pharmacopoeia (2020 edition) Part II "Amikacin"

Figure 4: Comparison data between RRT values obtained from experimental calculations and RRT values specified in the pharmacopoeia
After testing, it was found that the main peak area of sample RM-A131122 (RRT 0.32) did not change significantly during 24 hours of storage in acidic, neutral, and alkaline solutions, and the relative standard deviation was less than 2.0%. Therefore, it can be considered that this sample is relatively stable in the acidic, neutral and alkaline solution for 24 hours. The test data of this sample in different pH solutions are summarized as follows:

Figure 5: RM-A131122, Summary of Solution Stability Data
Blue color:acidic; Orange color: neutral ; Yellow color:alkaline
When the samples RM-A131101 (impurity A) and RM-A131106 (impurity F) were placed in acidic and neutral solutions for 24 hours, the main peak area did not change significantly, and the relative standard deviation was less than 2.0%. However, during the process of samples in alkaline solution for 24 hours, the main peak area changed greatly, and the relative standard deviation was greater than 2.0% (the relative standard deviation of impurity A was 2.73%, and the relative standard deviation of impurity F was 4.59%), and the impurity increased significantly. So these two samples are relatively stable in acidic and neutral solutions, and will undergo slow degradation in alkaline solutions. The test data of these 2 samples in solution environments with different pH values are summarized as follows:

Figure 6: RM-A131101 Summary of Solution Stability Data
Blue color:acidic; Orange color: neutral ; Yellow color:alkaline

Figure 7: RM-A131101 3D plot of solution stability data

Figure 8: RM-A131106 Summary of Solution Stability Data
Blue color:acidic; Orange color: neutral ; Yellow color:alkaline

Figure 9:RM-A131106 3D plot of solution stability data
Summary
In summary, through this experiment, we found that sample RM-A131122 (RRT 0.32) has good stability in acidic, neutral, and alkaline solutions. Samples RM-A131101 (Impurity A) and RM-A131106 (Impurity F) have good stability in acidic and neutral solutions, but poor stability in alkaline solutions, they will undergo slow degradation with prolonged storage time, attention should be paid during storage and testing, and it is recommended to prepare and use them as needed, they are not suitable as reserve solutions. If the stability data of the above 3 samples is needed, welcome to consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号