Time:2022-10-21
Introduction: This issue shares a case analysis of an abnormal batch of Dapoxetine R319140 impurity (QCS item number: RM-D162405) due to a process change.
Dapoxetine ( INN Dapoxetine, trade name: Priligy) is a fast acting selective serotonin reuptake inhibitor (SSRI), originally developed by Eli Lilly in the United States, and has been approved by countries such as China, Finland, Sweden, Portugal, Austria, Italy, and Spain. Considering the guiding role of imported drug registration standards in quality research, combined with the import registration standards of Dapoxetine hydrochloride tablets (Priligy) (registration number JX20150184, Figure 1), our center has synthesized and developed the five code impurities mentioned in the standards. The impurity code involved in this project are R320404, R320036, R316940, R319137, R319140(corresponding to QCS item number: RM-D162401, RM-D122404, RM-D162406, RM-D162404, RM-D162405).
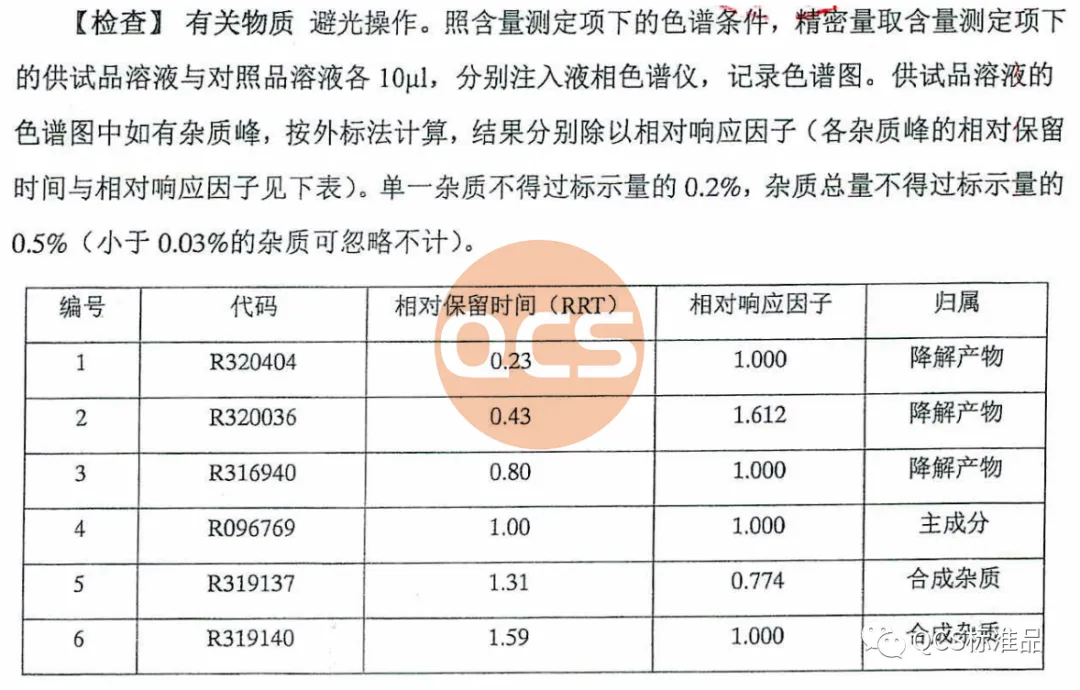
Figure 1: Source of impurity information for imported drug registration standard code: National Food and Drug Administration Import Drug Registration Standard (registration number JX20150184)
This issue shares an example of a quality after-sales issue with impurity R319140 (QCS item number: RM-D162405) caused by a process change.
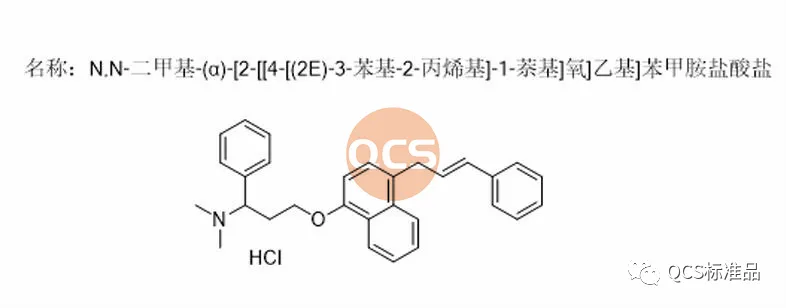
Figure 2: Registration standard number R319140 impurity structural formula (QCS item number: RM-D162405)
This product is the star product of Dapoxetine hydrochloride project, which has been produced and sold multiple times. However, the QCS Standard Substances Research Center encountered abnormal quality feedback when improving the production process of this product. This article will provide a detailed introduction to the research and development of the RM-D162405 product.
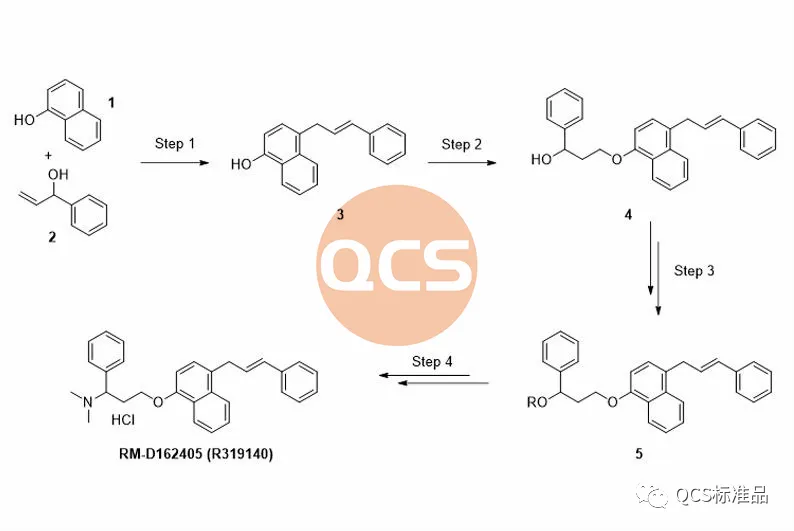
Figure 3: Product synthesis route of RM-D162405 (R319140) (Source: Synthesis Technology Department of QCS Standard Product Development Center)
During the preparation process of this product, the key intermediate styrenyl binaphthol 3 is generated, followed by alkylation and functional group modification to obtain the RM-D162405 product. In order to improve the reaction yield and upgrade the environmental protection of the process, our center continuously optimizes the products during the production process. For product RM-D162405, our center attempts to directly alkylate the key intermediate with chlorinated structure 6 to obtain the target product, and optimizes its synthesis route. It was found that mixing structures 3 and 6 under alkaline conditions will result in a structure that matches the target molecular weight, and the final product RM-D162405 with batch 17269 will be purified by chromatography.
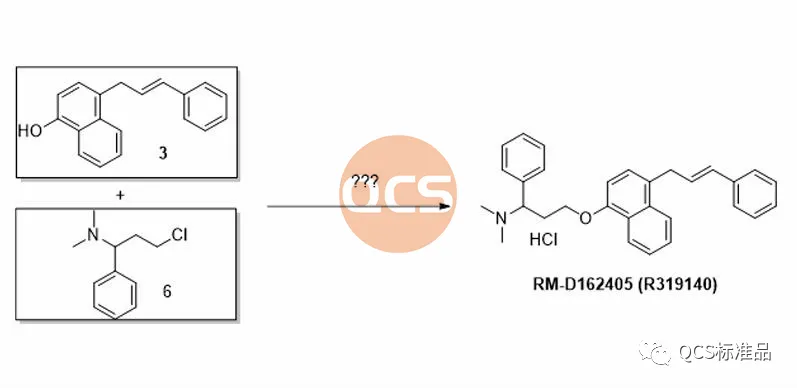
Figure 4: Attempt to synthesize intermediate of RM-D162405 (R319140) Product (Source: Synthesis Technology Department of QCS Standard Product Development Center)
The product was ultimately offered in the form of the target structure formate and the following data were obtained through HNMR spectroscopy detection:
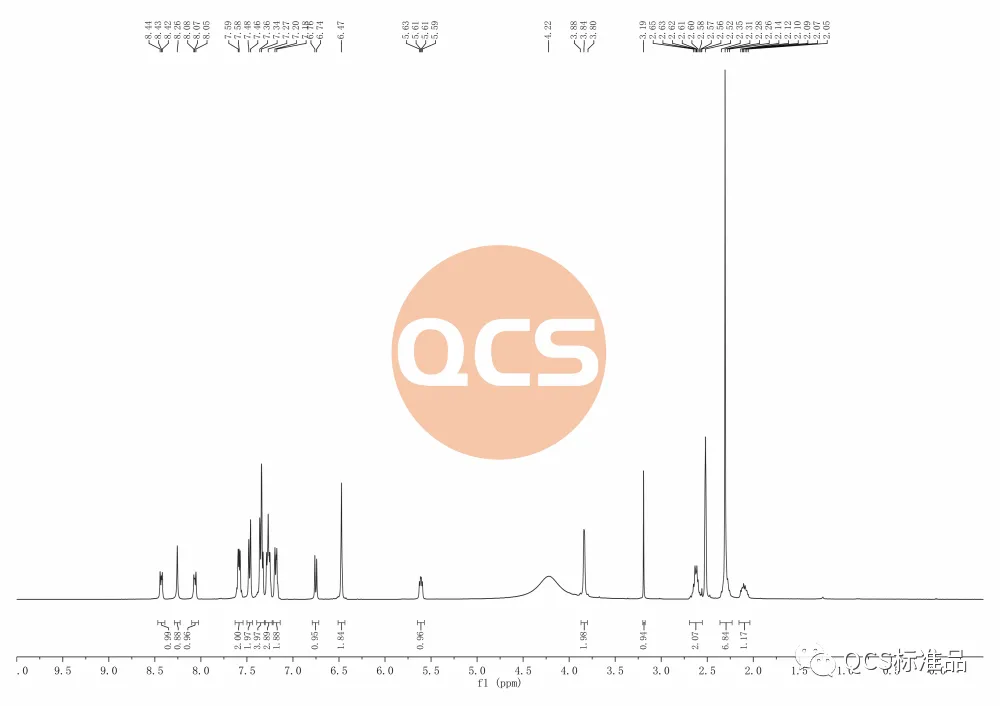
Figure 5: RM-D162405 Product Batch 17269 (solvent DMSO-d6, source: Analysis Technology Division, QCS Standard Development Center)
Analyzing the detection data, it can be found that the number of protons in the HNMR spectrum of this batch of products matches the target structure, and the number of aromatic regions and N-dimethyls are consistent with the target structure. However, the signal with a chemical shift in the 6-2ppm range is in an abnormal state. Due to the fact that the final form of this batch of products is in the form of formate, there may be differences from the reported hydrochloride form in literature, however, after converting the salt form of this batch of products to hydrochloride, there are still differences between the preliminary NMR detection data and theoretical data of RM-D162405 (R319140) impurities in batch 17269.
At the same time, our center received feedback from customers that there were differences between the liquid phase peak time (RT) and relative retention time (RRT) data of this batch of products and the file during the validation process using import registration standard method.
For this batch of products, the RRT data detected by the import registration standard method is 1.40, however, the RM-D162405 (R319140) product is marked with an RRT data of 1.59 in the import registration standard. At the same time, it was found that the liquid phase positioning of the 17269 batch of products did not match the pre process improvement batch retention samples during inter batch comparison. Therefore, combining NMR data and liquid phase differences, it can be determined that the process change has caused quality problems in the 17269 batch of products. In response to this quality issue, our center attaches great importance and immediately organizes scientific researchers to review the process and urgently arrange for the original process to resume production. After obtaining the product with batch number 20618, the product was immediately tested. The NMR data of the product obtained using the original process matched the historical data. Further comparison of literature NMR data and verification of import registration standard methods showed that the RRT of the resumed batch of products was consistent with the documents, and the product ultimately passed the customer's acceptance.(Interested users can contact the editor to obtain the correct product samples and MRI raw data packages)
(After process review, it was found that the intermediate structure underwent isomerization and rearrangement under the changed reaction conditions. Please contact the editor for detailed information)
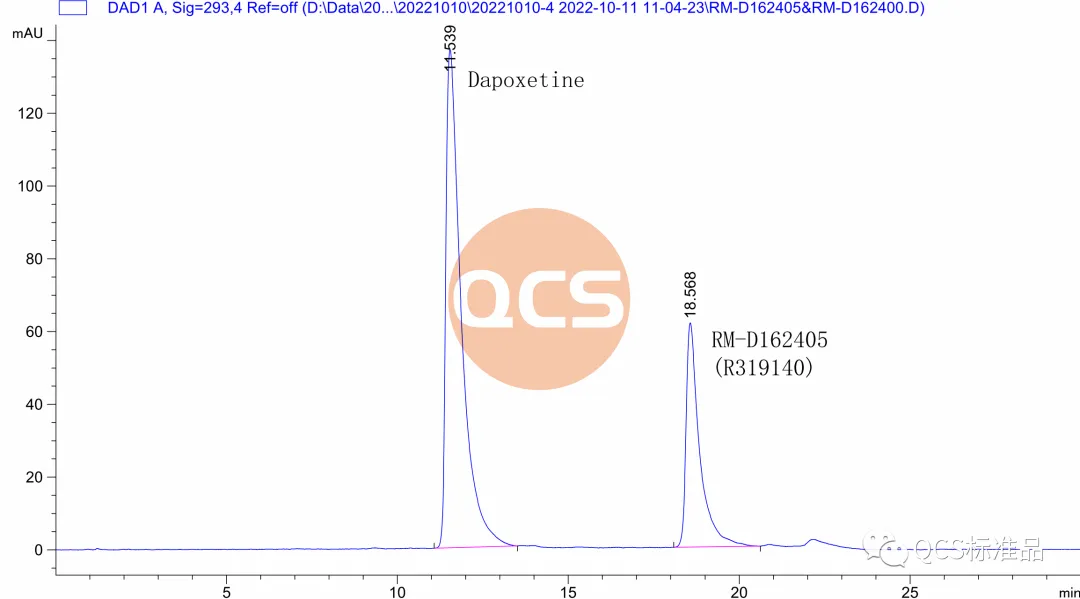
Figure 6: Liquid chromatography of product RM-D162405 batch 20618+dapoxetine hydrochloride (Source: Analysis Technology Department of QCS Standard Product Development Center)
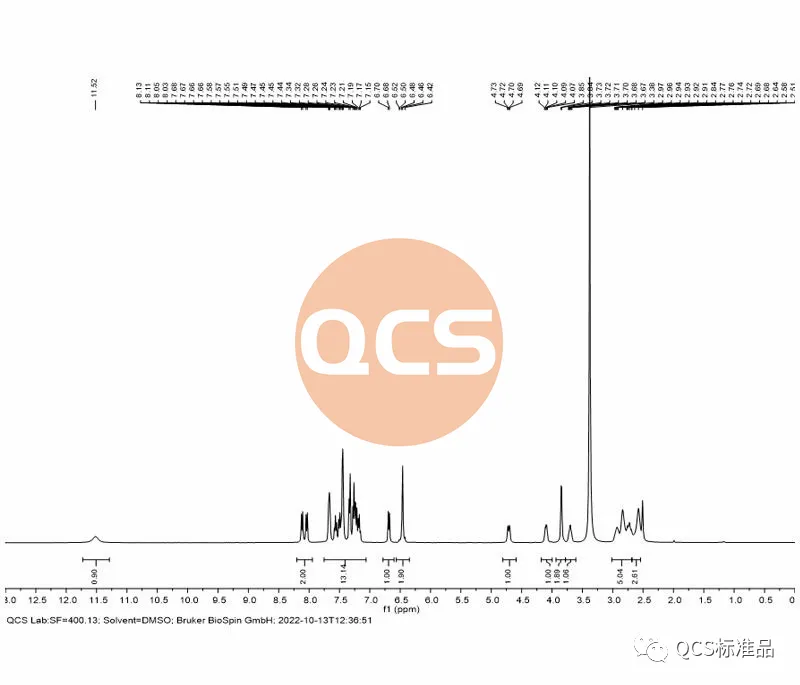
Figure 7: NMR spectrum of product RM-D162405 batch 20618 (Source: Analysis Technology Department of QCS Standard Product Development Center)
Unanswered mystery: Based on the synthesis process of Dapoxetine hydrochloride and potential side reactions in the reaction, our research center conducted theoretical analysis and process validation on impurities code R319137 (RM-D162404) and R319140 (RM-D162405). It has been confirmed that two impurities should exist in the following synthesis process and be generated through the following possible mechanisms:
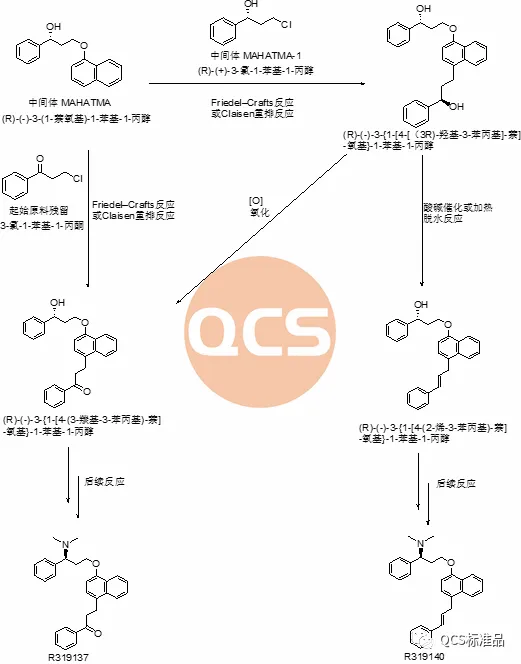
Figure 8: Synthesis Route 1 of Dapoxetine Hydrochloride and the Mechanism of Impurities R319137 (RM-D162404) and R319140 (RM-D162405) Production
However, further research on the synthesis process of dapoxetine hydrochloride has found that impurities R319137 (RM-D162404) and R319140 (RM-D162405) are widely present impurities in different synthesis processes. For example, our research center found in process research that impurities R319137 (RM-D162404) and R319140 (RM-D162405) are also generated in another synthesis process of Dapoxetine, as shown in the following figure. However, based on the analysis of the reaction mechanism, the path and cause of impurity generation in this process have not yet been obtained. Can readers and users help us answer this "unsolved mystery"?
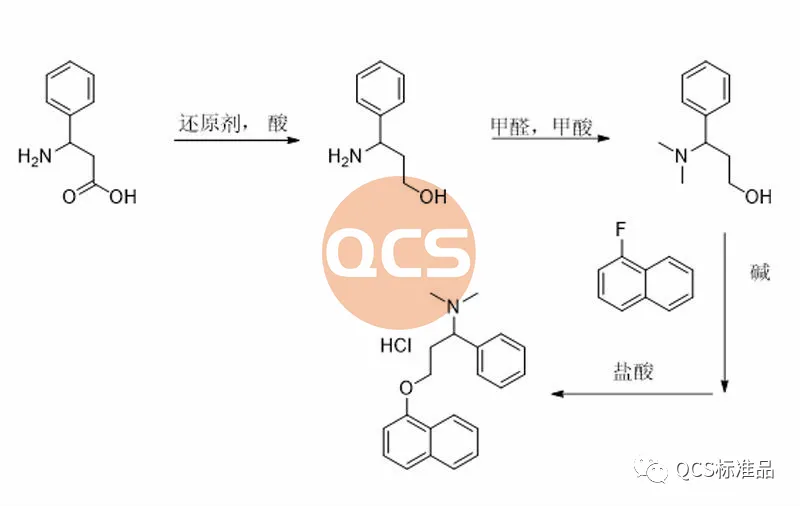
Figure 9: Synthesis route 2 of Dapoxetine hydrochloride
Welcome all teachers to communicate together or directly submit to our center. We are happy to share your knowledge with our clients.


Introduction: This issue shares a case analysis of an abnormal batch of Dapoxetine R319140 impurity (QCS item number: RM-D162405) due to a process change.
Dapoxetine ( INN Dapoxetine, trade name: Priligy) is a fast acting selective serotonin reuptake inhibitor (SSRI), originally developed by Eli Lilly in the United States, and has been approved by countries such as China, Finland, Sweden, Portugal, Austria, Italy, and Spain. Considering the guiding role of imported drug registration standards in quality research, combined with the import registration standards of Dapoxetine hydrochloride tablets (Priligy) (registration number JX20150184, Figure 1), our center has synthesized and developed the five code impurities mentioned in the standards. The impurity code involved in this project are R320404, R320036, R316940, R319137, R319140(corresponding to QCS item number: RM-D162401, RM-D122404, RM-D162406, RM-D162404, RM-D162405).

Figure 1: Source of impurity information for imported drug registration standard code: National Food and Drug Administration Import Drug Registration Standard (registration number JX20150184)
This issue shares an example of a quality after-sales issue with impurity R319140 (QCS item number: RM-D162405) caused by a process change.

Figure 2: Registration standard number R319140 impurity structural formula (QCS item number: RM-D162405)
This product is the star product of Dapoxetine hydrochloride project, which has been produced and sold multiple times. However, the QCS Standard Substances Research Center encountered abnormal quality feedback when improving the production process of this product. This article will provide a detailed introduction to the research and development of the RM-D162405 product.

Figure 3: Product synthesis route of RM-D162405 (R319140) (Source: Synthesis Technology Department of QCS Standard Product Development Center)
During the preparation process of this product, the key intermediate styrenyl binaphthol 3 is generated, followed by alkylation and functional group modification to obtain the RM-D162405 product. In order to improve the reaction yield and upgrade the environmental protection of the process, our center continuously optimizes the products during the production process. For product RM-D162405, our center attempts to directly alkylate the key intermediate with chlorinated structure 6 to obtain the target product, and optimizes its synthesis route. It was found that mixing structures 3 and 6 under alkaline conditions will result in a structure that matches the target molecular weight, and the final product RM-D162405 with batch 17269 will be purified by chromatography.

Figure 4: Attempt to synthesize intermediate of RM-D162405 (R319140) Product (Source: Synthesis Technology Department of QCS Standard Product Development Center)
The product was ultimately offered in the form of the target structure formate and the following data were obtained through HNMR spectroscopy detection:

Figure 5: RM-D162405 Product Batch 17269 (solvent DMSO-d6, source: Analysis Technology Division, QCS Standard Development Center)
Analyzing the detection data, it can be found that the number of protons in the HNMR spectrum of this batch of products matches the target structure, and the number of aromatic regions and N-dimethyls are consistent with the target structure. However, the signal with a chemical shift in the 6-2ppm range is in an abnormal state. Due to the fact that the final form of this batch of products is in the form of formate, there may be differences from the reported hydrochloride form in literature, however, after converting the salt form of this batch of products to hydrochloride, there are still differences between the preliminary NMR detection data and theoretical data of RM-D162405 (R319140) impurities in batch 17269.
At the same time, our center received feedback from customers that there were differences between the liquid phase peak time (RT) and relative retention time (RRT) data of this batch of products and the file during the validation process using import registration standard method.
For this batch of products, the RRT data detected by the import registration standard method is 1.40, however, the RM-D162405 (R319140) product is marked with an RRT data of 1.59 in the import registration standard. At the same time, it was found that the liquid phase positioning of the 17269 batch of products did not match the pre process improvement batch retention samples during inter batch comparison. Therefore, combining NMR data and liquid phase differences, it can be determined that the process change has caused quality problems in the 17269 batch of products. In response to this quality issue, our center attaches great importance and immediately organizes scientific researchers to review the process and urgently arrange for the original process to resume production. After obtaining the product with batch number 20618, the product was immediately tested. The NMR data of the product obtained using the original process matched the historical data. Further comparison of literature NMR data and verification of import registration standard methods showed that the RRT of the resumed batch of products was consistent with the documents, and the product ultimately passed the customer's acceptance.(Interested users can contact the editor to obtain the correct product samples and MRI raw data packages)
(After process review, it was found that the intermediate structure underwent isomerization and rearrangement under the changed reaction conditions. Please contact the editor for detailed information)

Figure 6: Liquid chromatography of product RM-D162405 batch 20618+dapoxetine hydrochloride (Source: Analysis Technology Department of QCS Standard Product Development Center)

Figure 7: NMR spectrum of product RM-D162405 batch 20618 (Source: Analysis Technology Department of QCS Standard Product Development Center)
Unanswered mystery: Based on the synthesis process of Dapoxetine hydrochloride and potential side reactions in the reaction, our research center conducted theoretical analysis and process validation on impurities code R319137 (RM-D162404) and R319140 (RM-D162405). It has been confirmed that two impurities should exist in the following synthesis process and be generated through the following possible mechanisms:

Figure 8: Synthesis Route 1 of Dapoxetine Hydrochloride and the Mechanism of Impurities R319137 (RM-D162404) and R319140 (RM-D162405) Production
However, further research on the synthesis process of dapoxetine hydrochloride has found that impurities R319137 (RM-D162404) and R319140 (RM-D162405) are widely present impurities in different synthesis processes. For example, our research center found in process research that impurities R319137 (RM-D162404) and R319140 (RM-D162405) are also generated in another synthesis process of Dapoxetine, as shown in the following figure. However, based on the analysis of the reaction mechanism, the path and cause of impurity generation in this process have not yet been obtained. Can readers and users help us answer this "unsolved mystery"?

Figure 9: Synthesis route 2 of Dapoxetine hydrochloride
Welcome all teachers to communicate together or directly submit to our center. We are happy to share your knowledge with our clients.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号