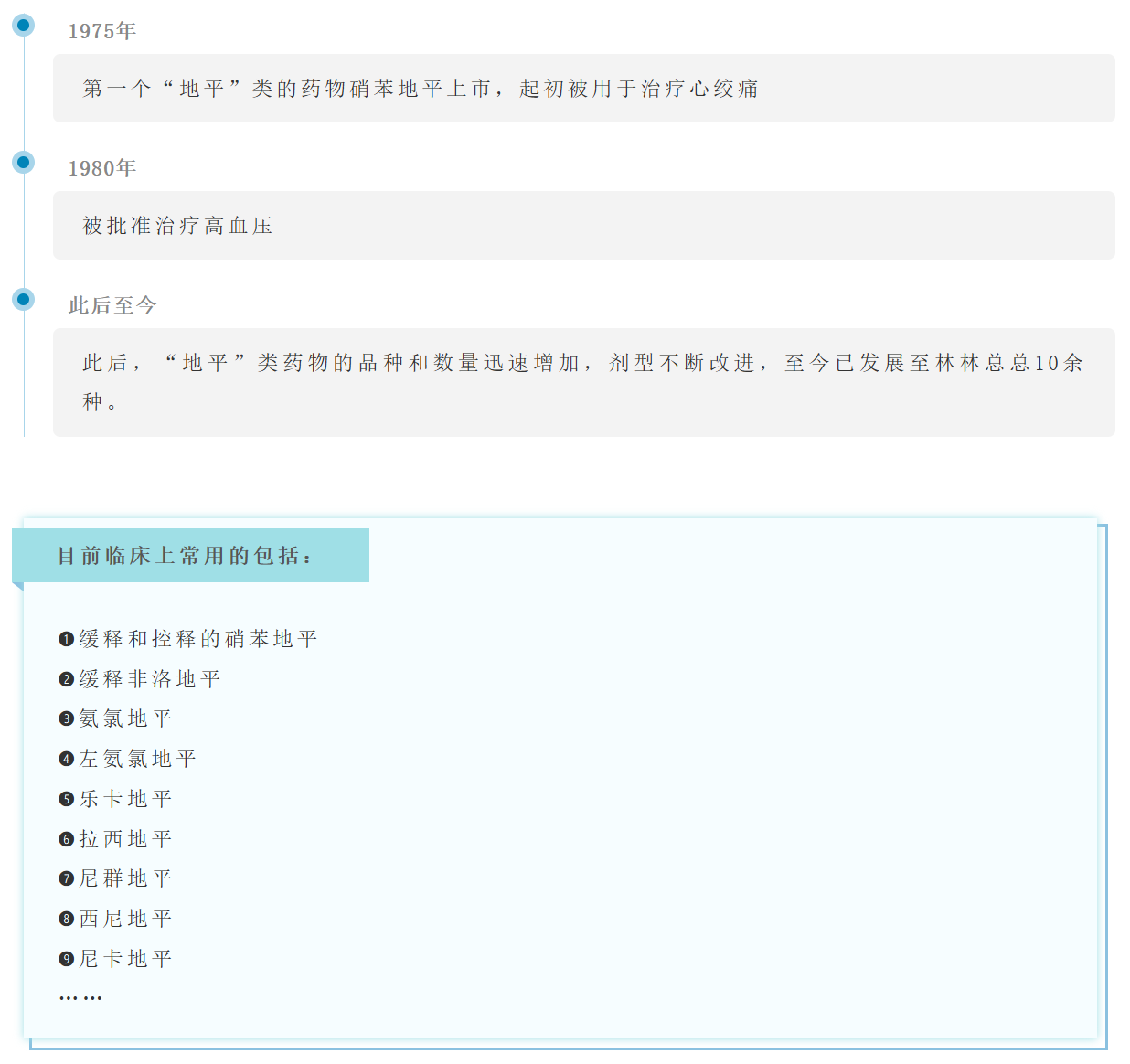
Time:2024-06-27
Dihydropyridine calcium channel blockers, commonly known as "dihydropyridine" antihypertensive drugs, are the most widely used antihypertensive drugs for patients with hypertension in our country. There are many members of the "dihydropyridine" class of drugs. The general structural formula of the various dihydropyridine drugs is shown below.
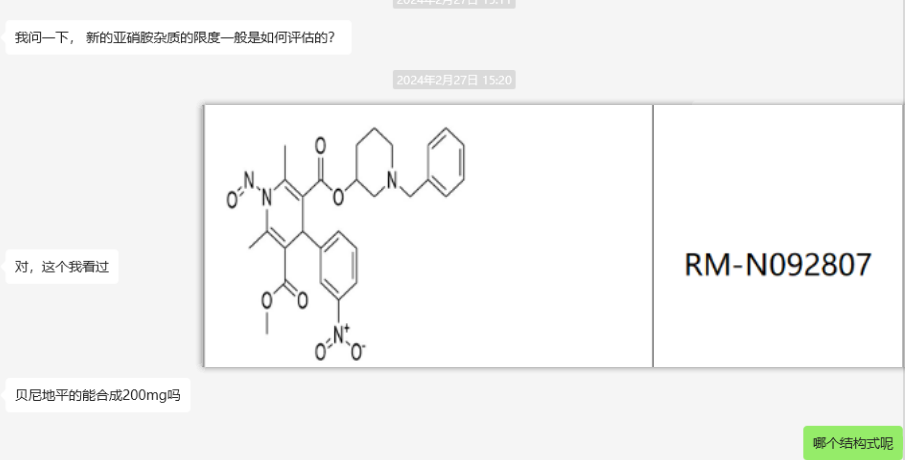
As an antihypertensive drug, will the research on impurities of dihydropyridine drugs, especially the research on N-nitrosamine impurities, cause your blood pressure to rise instead of fall as a researcher?
The research on N-nitrosamine impurities is becoming increasingly strict. For the research on N-nitrosamine impurities of dihydrochloride drugs, have you ever encountered the difficulty in finding N-nitrosamine impurities or the N-nitrosamine impurities are not the same as the ones on the label? This article will help you answer your questions and help you sort out the correct research methods for dihydrochloride N-nitrosamine impurities.
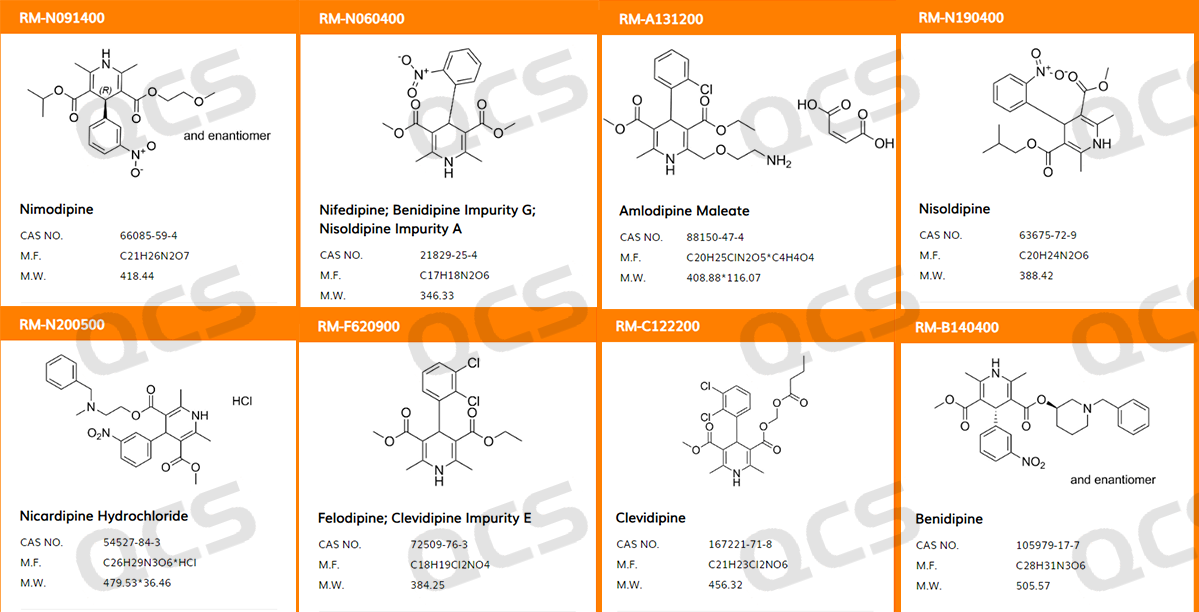
Figure 1: Collection of Horizontal Structures (Excerpt)
#01 Nitrosamines from the tertiary system: a case that only exists in theory
As dihydropyridine calcium channel blockers, dihydropyridine drugs have a reaction site in their chemical structure that can "theoretically" undergo nitrosamination. Therefore, the "theoretically" existing nitrosamine impurities must be studied to assess their risks.
In the study of N-nitrosamine impurities, various regulatory agencies have introduced different evaluation methods. For example, EMA classified N-nitrosamine impurities based on whether they have ortho-protons, ortho-quaternary carbons, and potency index in its Carcinogenic Potency Categorization Approach (CPCA).
In the EMA document "Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal product", felodipine was selected as a case to demonstrate the evaluation process, and the so-called felodipine nitrosamine impurities were classified as Category 5.
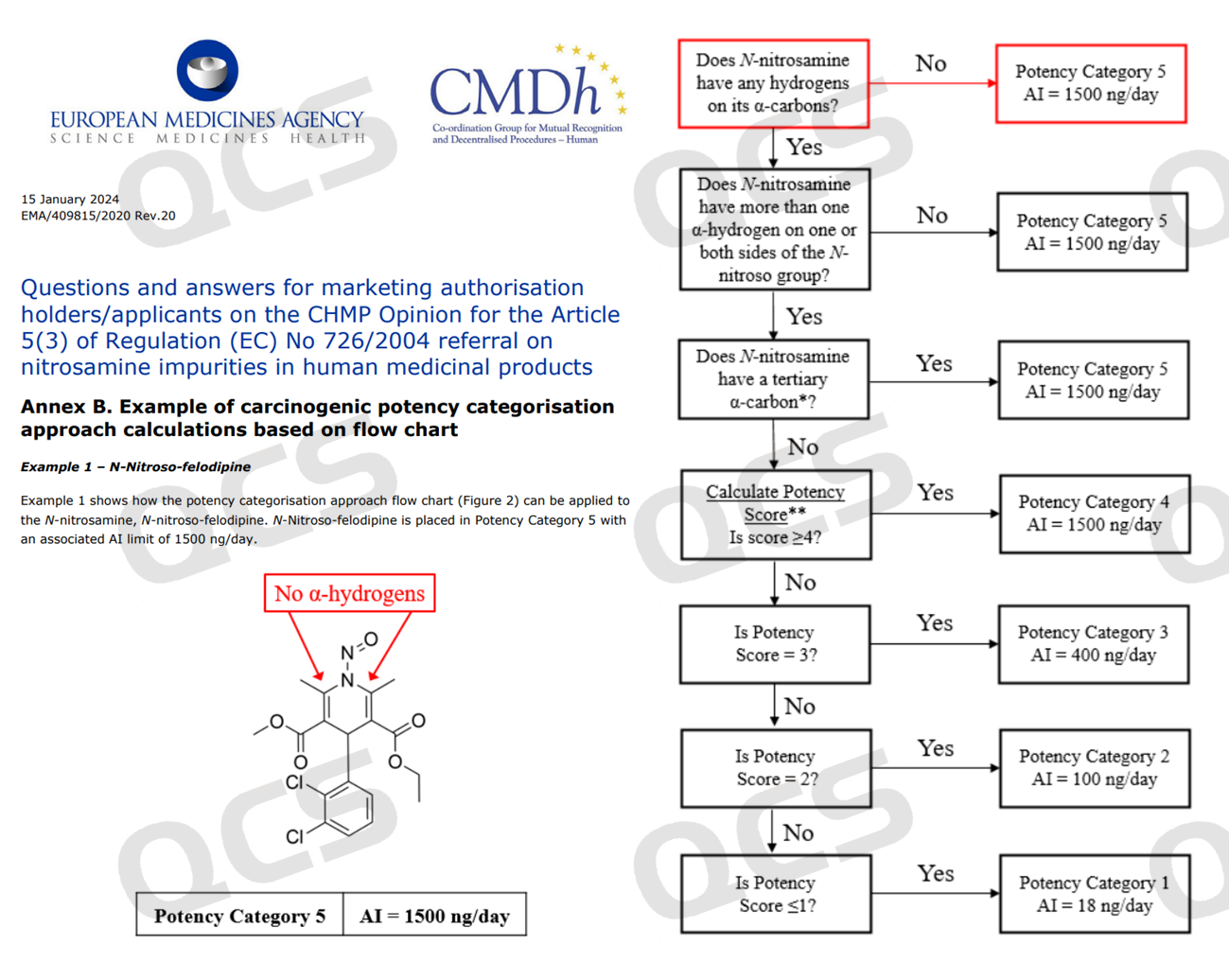
Figure 2: EMA potencalculation examples (Calculation of potency score)
However, only researchers who have actually studied this variety will truly understand the "harm" of nitrosamine impurities in Felodipine.
In many official guidelines, the evaluation of N-nitrosamine impurities is mostly based on theoretical calculations or simulation estimates, and the possibility of the existence of impurities under real-world conditions is not evaluated. The laboratory of the QCS Reference Material Research and Development Center has attempted to synthesize and prepare nitrosamine impurities of multiple dipine drugs. However, we regretfully discovered that dipine-structured nitrosamine impurities cannot exist stably. For example, in our laboratory's attempt to synthesize nitrosamine impurities in nimodipine, we regretfully discovered that under many nitrosamine synthesis conditions, reactions with it could not occur, and only products with oxidative aromatization of the dipine structure could be obtained. Even under the conditions of classic nitrosamination, when we reacted the nimodipine API with sodium nitrite, only the signal of the aromatized structure Nimodipine EP Impurity A (QCS Product No. RM-N131501, CSA No. 85677-93-6) could be observed. The above experimental results have been reproduced in different dipine products, and the results are consistent with historical literature data.
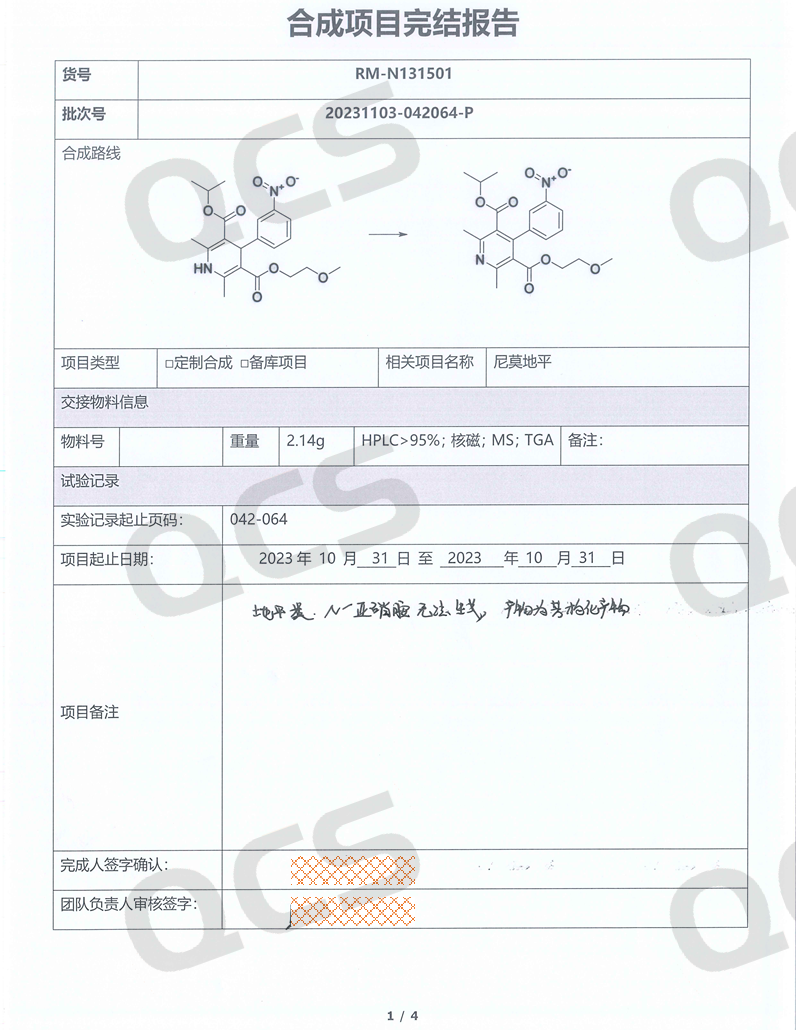
Figure 3: QCS R&D Center Nimodipine Nitrosamine Synthesis Report

Figure 4: QCS R&D Center Nimodipine Nitrosamination Test Record
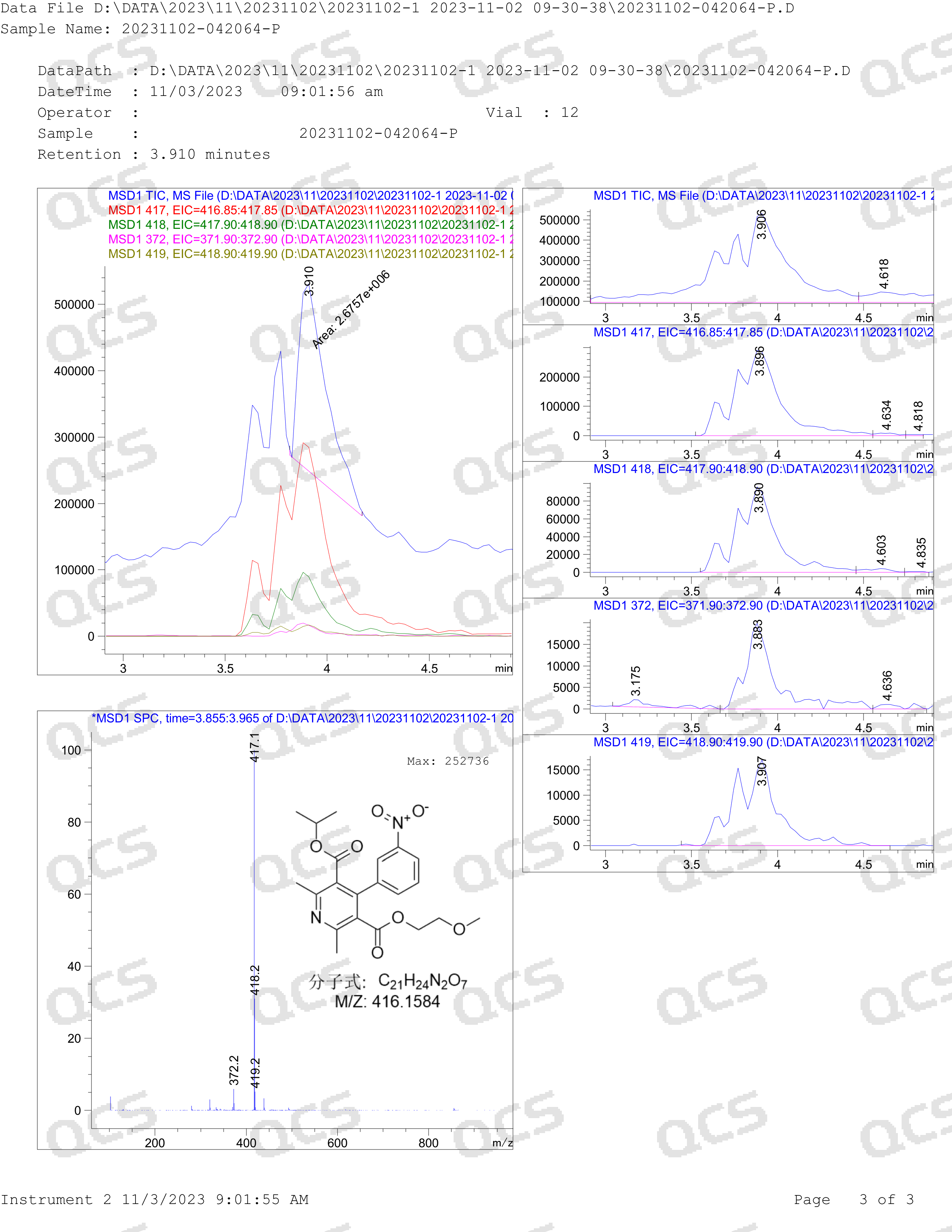
Figure 5: QCS R&D Center Nimodipine Nitrosamination LCMS Data
References:
3. Gillat et al. (1984 - DOI: 10.1016/0278-6915(84)90005-x)
参考自:USP Nitrosamines Exchange
Reference from: USP Nitrosamines Exchange
Therefore, for the study of N-nitrosamine impurities, it is necessary to add a "0" step in the research scheme: whether the compound is stable or can be synthesized.
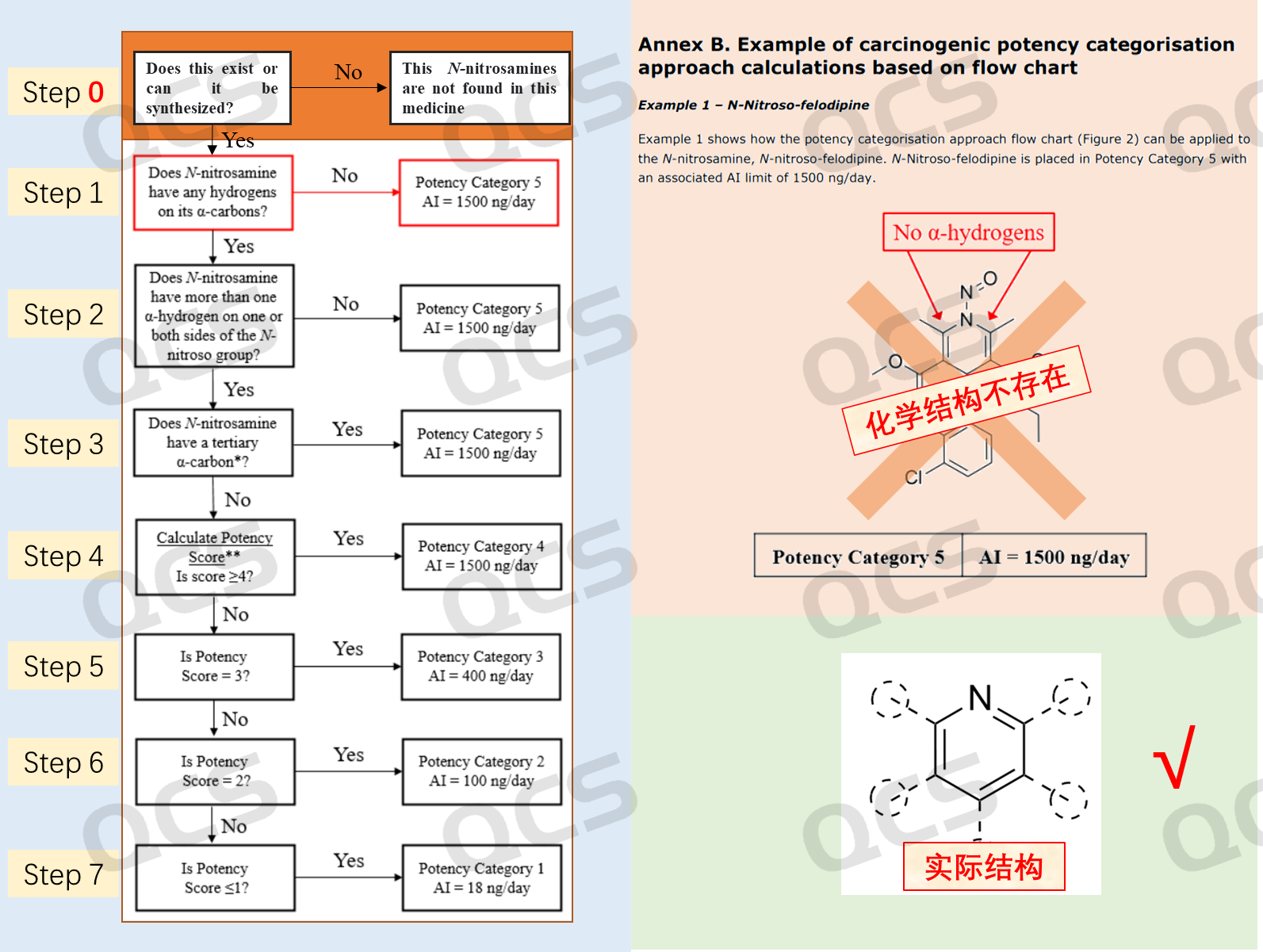
Figure 6: QCS nitrosamine evaluation flow chart optimization
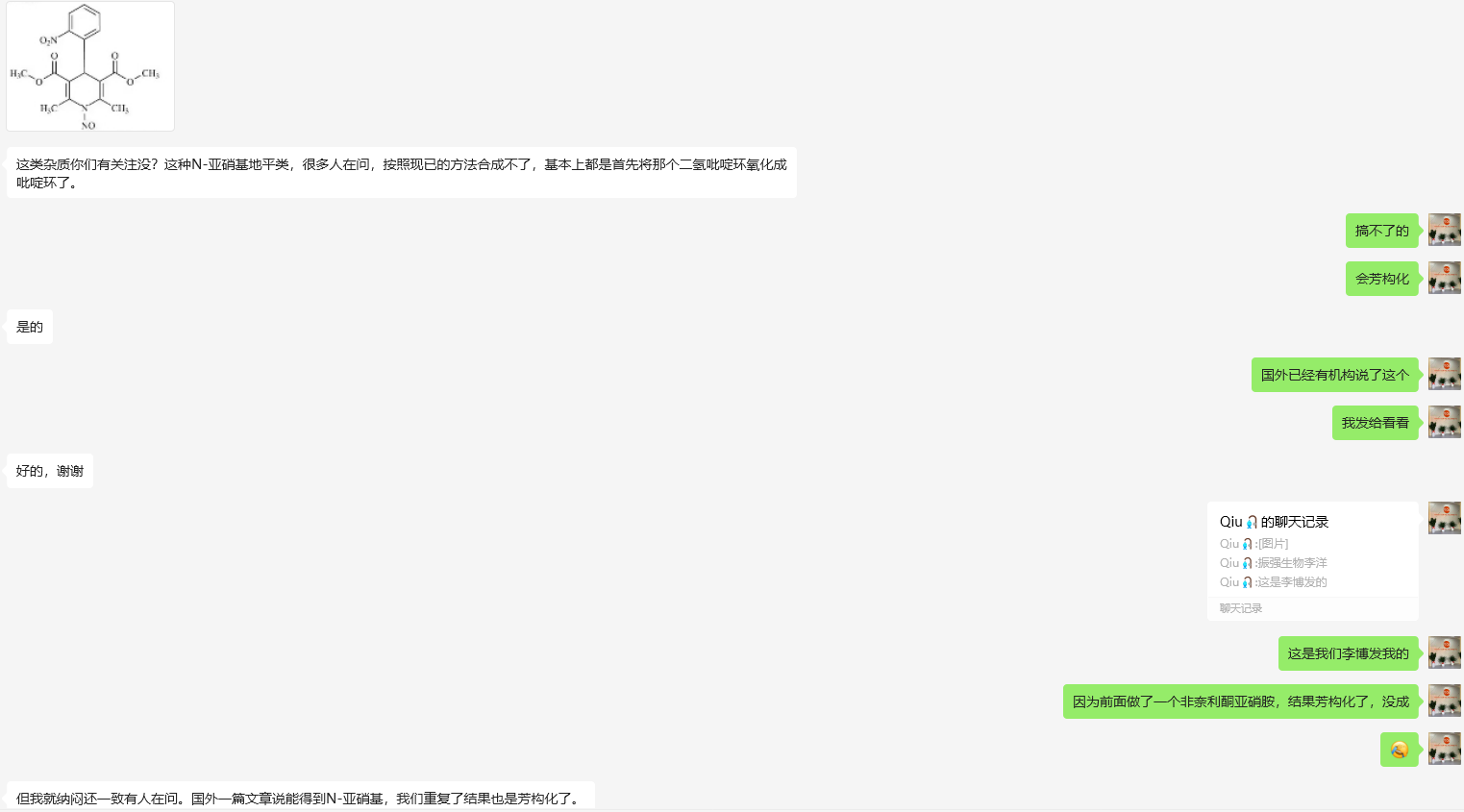
Many institutions or groups have published opinions and conclusions on the study of nitrosamine impurities in diping compounds that the impurities cannot exist or cannot be synthesized.
relevant document
EFPIA Position Paper
For example, there have been many feedbacks about such nitrosamine impurities in the USP Nitrosamine Exchange Forum, and the European Federation of Pharmaceutical Industries and Associations (EFPIA) has published a position paper similar to the industry consensus, which expresses the view that nitrosamines cannot be stable and cannot exist. We believe that the nitrosamine impurities of dihydropyridine drug molecules are unavailable, and the so-called dihydropyridine impurity samples on the market are all erroneous products of aromatization.

Figure 7: Screenshot of USP and EFPIA industry consensus documents
#02N-Nitrosamine Potential Assessment Method Alternative Standard Comparison Method
Therefore, for researchers of dihydropyridine drugs, we recommend the use of a reaction possibility assessment strategy to replace the impossible standard sample control strategy. That is, by placing the drug molecule under standard nitrosamination synthesis reaction conditions (such as high concentrations of sodium nitrite and acid), it is proved that N-nitrosamine impurities will not be generated even under extremely strong nitrosamination conditions, thereby completing the evaluation of nitrosamine impurities in drug products.
QCS Reference Material Research and Development Center believes that the following dihydropyridine nitrosamine impurities (Figure 8) cannot be synthesized, and recommends the use of a reaction possibility assessment strategy in related projects.
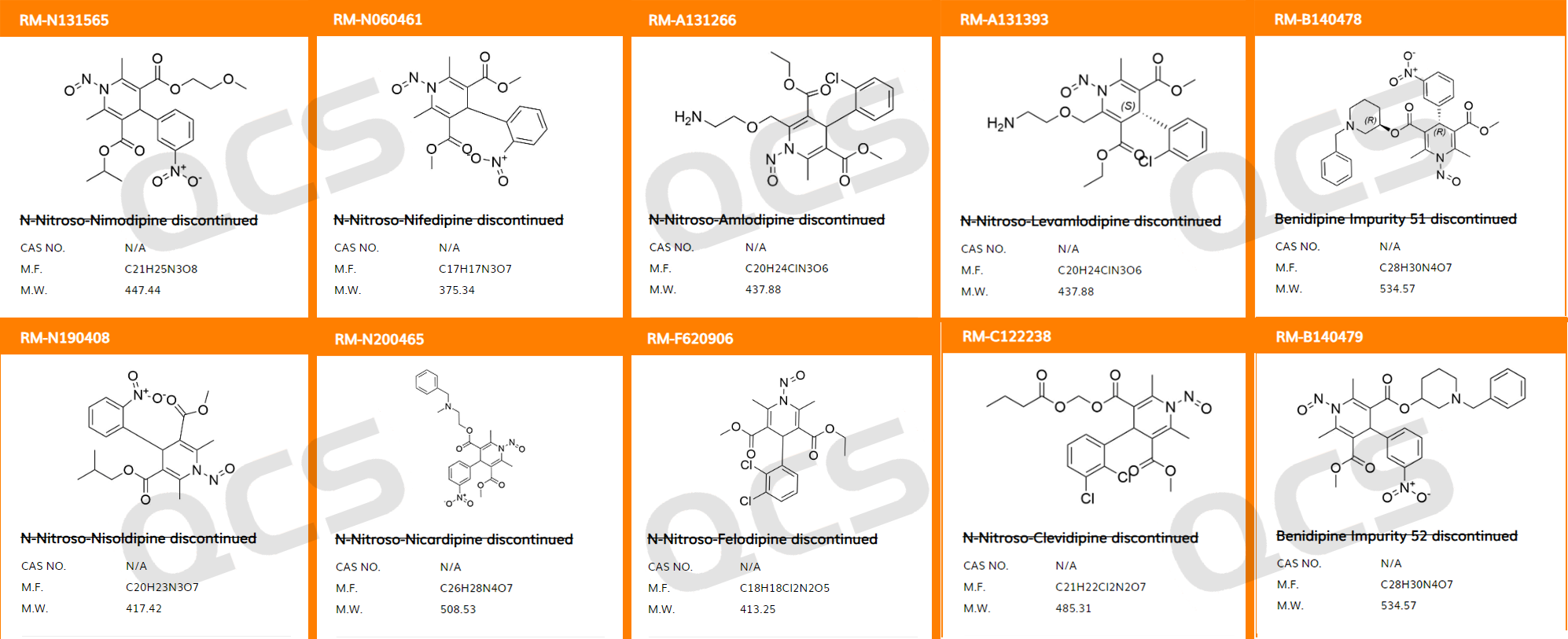
Figure 8: A series of dihydronitrosamine impurities that cannot be synthesized
We aim to leverage our experimental data and technical expertise to offer customers valuable data support and theoretical guidance. Simultaneously, we aspire to minimize the pitfalls for our peers and contribute to the advancement of quality research and standard material development in Chinese medicine. (For relevant literature and industry consensus documents, please reach out to our dedicated sales team.)






Dihydropyridine calcium channel blockers, commonly known as "dihydropyridine" antihypertensive drugs, are the most widely used antihypertensive drugs for patients with hypertension in our country. There are many members of the "dihydropyridine" class of drugs. The general structural formula of the various dihydropyridine drugs is shown below.
As an antihypertensive drug, will the research on impurities of dihydropyridine drugs, especially the research on N-nitrosamine impurities, cause your blood pressure to rise instead of fall as a researcher?

The research on N-nitrosamine impurities is becoming increasingly strict. For the research on N-nitrosamine impurities of dihydrochloride drugs, have you ever encountered the difficulty in finding N-nitrosamine impurities or the N-nitrosamine impurities are not the same as the ones on the label? This article will help you answer your questions and help you sort out the correct research methods for dihydrochloride N-nitrosamine impurities.

Figure 1: Collection of Horizontal Structures (Excerpt)
#01 Nitrosamines from the tertiary system: a case that only exists in theory
As dihydropyridine calcium channel blockers, dihydropyridine drugs have a reaction site in their chemical structure that can "theoretically" undergo nitrosamination. Therefore, the "theoretically" existing nitrosamine impurities must be studied to assess their risks.
In the study of N-nitrosamine impurities, various regulatory agencies have introduced different evaluation methods. For example, EMA classified N-nitrosamine impurities based on whether they have ortho-protons, ortho-quaternary carbons, and potency index in its Carcinogenic Potency Categorization Approach (CPCA).
In the EMA document "Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal product", felodipine was selected as a case to demonstrate the evaluation process, and the so-called felodipine nitrosamine impurities were classified as Category 5.

Figure 2: EMA potencalculation examples (Calculation of potency score)
However, only researchers who have actually studied this variety will truly understand the "harm" of nitrosamine impurities in Felodipine.
In many official guidelines, the evaluation of N-nitrosamine impurities is mostly based on theoretical calculations or simulation estimates, and the possibility of the existence of impurities under real-world conditions is not evaluated. The laboratory of the QCS Reference Material Research and Development Center has attempted to synthesize and prepare nitrosamine impurities of multiple dipine drugs. However, we regretfully discovered that dipine-structured nitrosamine impurities cannot exist stably. For example, in our laboratory's attempt to synthesize nitrosamine impurities in nimodipine, we regretfully discovered that under many nitrosamine synthesis conditions, reactions with it could not occur, and only products with oxidative aromatization of the dipine structure could be obtained. Even under the conditions of classic nitrosamination, when we reacted the nimodipine API with sodium nitrite, only the signal of the aromatized structure Nimodipine EP Impurity A (QCS Product No. RM-N131501, CSA No. 85677-93-6) could be observed. The above experimental results have been reproduced in different dipine products, and the results are consistent with historical literature data.

Figure 3: QCS R&D Center Nimodipine Nitrosamine Synthesis Report

Figure 4: QCS R&D Center Nimodipine Nitrosamination Test Record

Figure 5: QCS R&D Center Nimodipine Nitrosamination LCMS Data
References:
3. Gillat et al. (1984 - DOI: 10.1016/0278-6915(84)90005-x)
参考自:USP Nitrosamines Exchange
Reference from: USP Nitrosamines Exchange
Therefore, for the study of N-nitrosamine impurities, it is necessary to add a "0" step in the research scheme: whether the compound is stable or can be synthesized.

Figure 6: QCS nitrosamine evaluation flow chart optimization
Many institutions or groups have published opinions and conclusions on the study of nitrosamine impurities in diping compounds that the impurities cannot exist or cannot be synthesized.
relevant document
EFPIA Position Paper
For example, there have been many feedbacks about such nitrosamine impurities in the USP Nitrosamine Exchange Forum, and the European Federation of Pharmaceutical Industries and Associations (EFPIA) has published a position paper similar to the industry consensus, which expresses the view that nitrosamines cannot be stable and cannot exist. We believe that the nitrosamine impurities of dihydropyridine drug molecules are unavailable, and the so-called dihydropyridine impurity samples on the market are all erroneous products of aromatization.

Figure 7: Screenshot of USP and EFPIA industry consensus documents
#02N-Nitrosamine Potential Assessment Method Alternative Standard Comparison Method
Therefore, for researchers of dihydropyridine drugs, we recommend the use of a reaction possibility assessment strategy to replace the impossible standard sample control strategy. That is, by placing the drug molecule under standard nitrosamination synthesis reaction conditions (such as high concentrations of sodium nitrite and acid), it is proved that N-nitrosamine impurities will not be generated even under extremely strong nitrosamination conditions, thereby completing the evaluation of nitrosamine impurities in drug products.
QCS Reference Material Research and Development Center believes that the following dihydropyridine nitrosamine impurities (Figure 8) cannot be synthesized, and recommends the use of a reaction possibility assessment strategy in related projects.

Figure 8: A series of dihydronitrosamine impurities that cannot be synthesized
We aim to leverage our experimental data and technical expertise to offer customers valuable data support and theoretical guidance. Simultaneously, we aspire to minimize the pitfalls for our peers and contribute to the advancement of quality research and standard material development in Chinese medicine. (For relevant literature and industry consensus documents, please reach out to our dedicated sales team.)


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号