Time:2024-05-22
01 Introduction
Today we will share the study on the stability of specific impurities in therapeutic drug for renal anemia Roxadustat. Roxadustat (code name FG-4592) capsule is the world's first developed small molecule hypoxia inducible factor proline hydroxylase inhibitors (HIF-PHI) for the treatment of renal anemia. It can inhibit the PH enzyme by simulating one of the substrates of proline hydroxylase (PH), ketoglutarate, and affect the role of PH enzyme in maintaining a balance of HIF generation and degradation rate, thereby achieving the goal of correcting anemia.
02 Experimental plan
In this experiment, our center conducted a solution stability study on three code impurities of Roxadustat using chromatographic conditions used in the imported formulation method. The sample product number, code, and structural formula used are shown in Figures 1 and 2, and the method information is shown in Figure 3
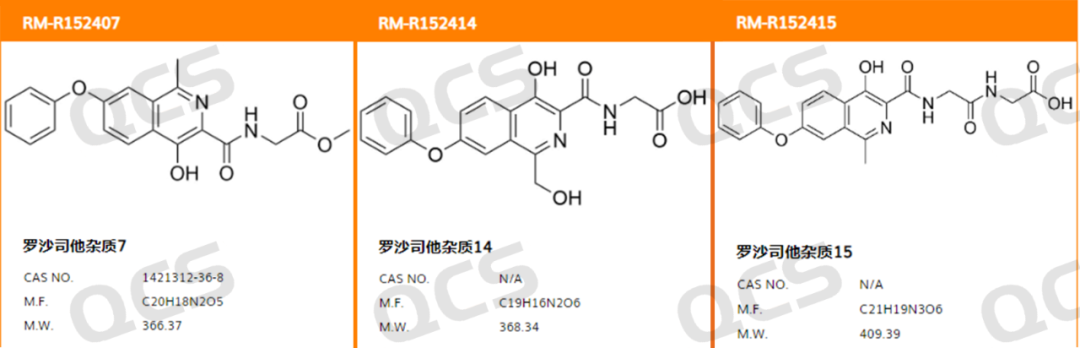
Figure 1: The impurity item number and structural formula in this study
(source: QCS Standard Material Research and Development Center)
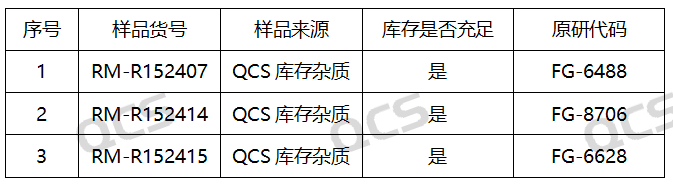
Figure 2: Corresponding Relationship between Impurity Item Number and Impurity Code
(Source: QCS Standard Material Research and Development Center)
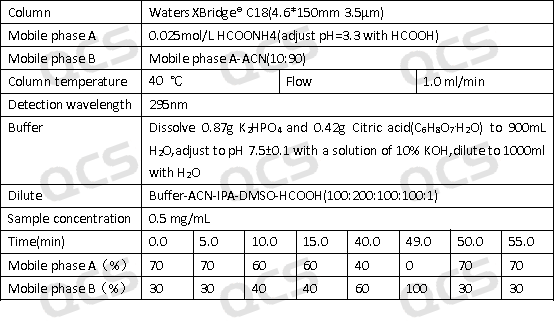
Figure 3: Stability experiment chromatographic conditions
(source: QCS standard substance research and development center)
In this experiment, the experimenter took an appropriate amount of RM-R152407 (code FG-6488), RM-R152414 (code FG-8706), and RM-R152415 (code FG-6628) each and placed them in acidic, neutral, and alkaline solutions. They were placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively, and then injected samples according to the chromatographic conditions in Figure 3 for detection. The changes in the peak area of the main peak in the chromatogram were observed as the sample solution was placed for an extended period of time, and the stability of the sample solution was determined based on this.
03 Empirical conclusion
After testing, it was found that the main peak area of samples RM-R152414 (code FG-8706) and RM-R152415 (code FG-6628) did not change much during 24 hours of storage in acidic, neutral, and alkaline solutions, with relative standard deviations less than 2.0%. It can be considered that samples RM-R152414 (code FG-8706) and RM-R152415 (code FG-6628) were relatively stable after 24 hours of storage in acidic, neutral, and alkaline solutions. While the main peak area of the sample RM-R152407 (code FG-6488) did not change much during 24 hours of storage in acidic and neutral solutions, with relative standard deviations less than 2.0%, after 24 hours of storage in alkaline solutions, the peak area of the main peak changed significantly and impurities increase significantly. Therefore, sample RM-R152407 (code FG-6488) is relatively stable in acidic and neutral solutions, but it undergoes slow degradation in alkaline solutions.
The detection data of samples RM-R152407 (code FG-6488), RM-R152414 (code FG-8706), and RM-R152415 (code FG-6628) under different pH conditions are summarized as follows:
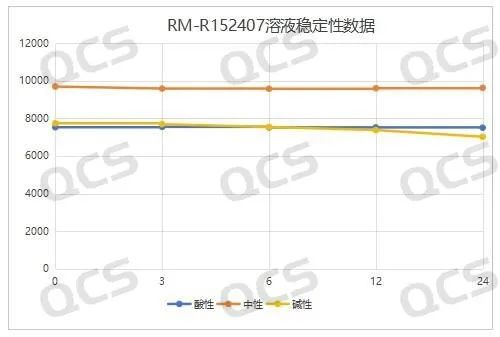
acidic, neutral, alkaline
Figure 4: Summary of Stability Data for RM-R152407 (Code FG-6488) Solution
(Source: QCS Standard Material Research and Development Center)
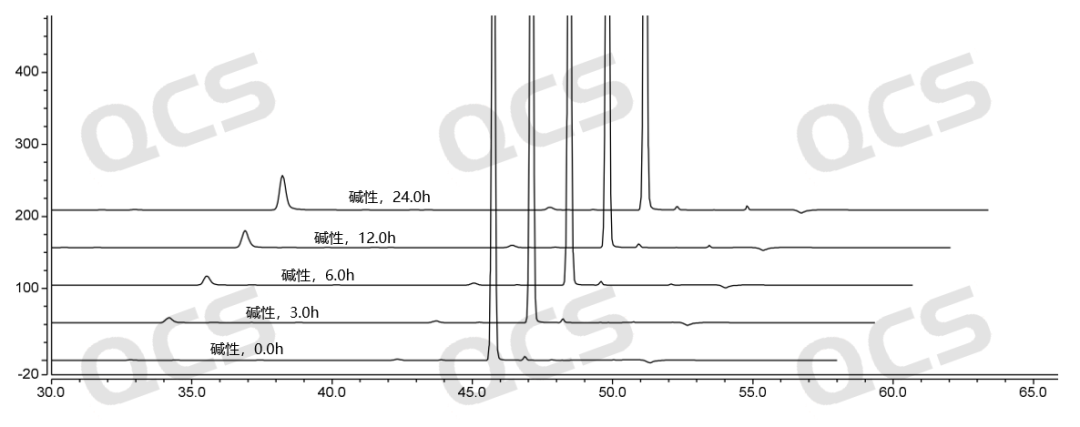
Figure 5: Stability data of RM-R152407 (code FG-6488) solution under alkaline conditions
(source: QCS Standard Material Research and Development Center)
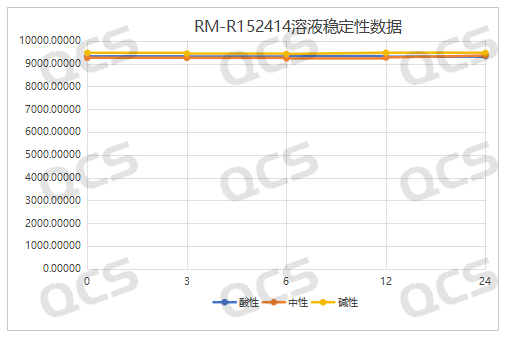
acidic, neutral, alkaline
Figure 6: Summary of Solution Stability Data for RM-R152414 (Code FG-8706)
(Source: QCS Reference Material Research and Development Center)
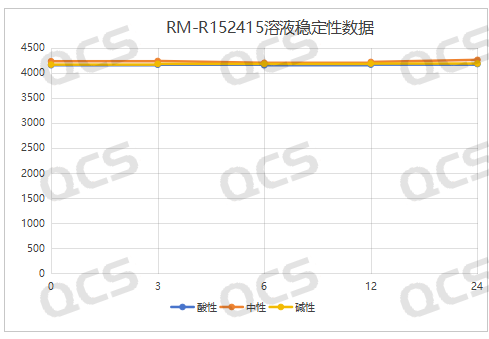
acidic, neutral, alkaline
Figure 7: Summary of Solution Stability Data for RM-R152415 (Code FG-6628)
(Source: QCS Reference Material Research and Development Center)
04 summary
In summary, through this experiment, we found that samples RM-R152414 (code FG-8706) and RM-R152415 (code FG-6628) have good stability. Sample RM-R152407 (code FG-6488) has good stability in acidic and neutral solutions, but poor stability in alkaline solutions. Attention should be paid to storage and detection processes. If you need stability data for the above three samples, welcome to consult our company.


01 Introduction
Today we will share the study on the stability of specific impurities in therapeutic drug for renal anemia Roxadustat. Roxadustat (code name FG-4592) capsule is the world's first developed small molecule hypoxia inducible factor proline hydroxylase inhibitors (HIF-PHI) for the treatment of renal anemia. It can inhibit the PH enzyme by simulating one of the substrates of proline hydroxylase (PH), ketoglutarate, and affect the role of PH enzyme in maintaining a balance of HIF generation and degradation rate, thereby achieving the goal of correcting anemia.
02 Experimental plan
In this experiment, our center conducted a solution stability study on three code impurities of Roxadustat using chromatographic conditions used in the imported formulation method. The sample product number, code, and structural formula used are shown in Figures 1 and 2, and the method information is shown in Figure 3

Figure 1: The impurity item number and structural formula in this study
(source: QCS Standard Material Research and Development Center)

Figure 2: Corresponding Relationship between Impurity Item Number and Impurity Code
(Source: QCS Standard Material Research and Development Center)

Figure 3: Stability experiment chromatographic conditions
(source: QCS standard substance research and development center)
In this experiment, the experimenter took an appropriate amount of RM-R152407 (code FG-6488), RM-R152414 (code FG-8706), and RM-R152415 (code FG-6628) each and placed them in acidic, neutral, and alkaline solutions. They were placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively, and then injected samples according to the chromatographic conditions in Figure 3 for detection. The changes in the peak area of the main peak in the chromatogram were observed as the sample solution was placed for an extended period of time, and the stability of the sample solution was determined based on this.
03 Empirical conclusion
After testing, it was found that the main peak area of samples RM-R152414 (code FG-8706) and RM-R152415 (code FG-6628) did not change much during 24 hours of storage in acidic, neutral, and alkaline solutions, with relative standard deviations less than 2.0%. It can be considered that samples RM-R152414 (code FG-8706) and RM-R152415 (code FG-6628) were relatively stable after 24 hours of storage in acidic, neutral, and alkaline solutions. While the main peak area of the sample RM-R152407 (code FG-6488) did not change much during 24 hours of storage in acidic and neutral solutions, with relative standard deviations less than 2.0%, after 24 hours of storage in alkaline solutions, the peak area of the main peak changed significantly and impurities increase significantly. Therefore, sample RM-R152407 (code FG-6488) is relatively stable in acidic and neutral solutions, but it undergoes slow degradation in alkaline solutions.
The detection data of samples RM-R152407 (code FG-6488), RM-R152414 (code FG-8706), and RM-R152415 (code FG-6628) under different pH conditions are summarized as follows:

acidic, neutral, alkaline
Figure 4: Summary of Stability Data for RM-R152407 (Code FG-6488) Solution
(Source: QCS Standard Material Research and Development Center)

Figure 5: Stability data of RM-R152407 (code FG-6488) solution under alkaline conditions
(source: QCS Standard Material Research and Development Center)

acidic, neutral, alkaline
Figure 6: Summary of Solution Stability Data for RM-R152414 (Code FG-8706)
(Source: QCS Reference Material Research and Development Center)

acidic, neutral, alkaline
Figure 7: Summary of Solution Stability Data for RM-R152415 (Code FG-6628)
(Source: QCS Reference Material Research and Development Center)
04 summary
In summary, through this experiment, we found that samples RM-R152414 (code FG-8706) and RM-R152415 (code FG-6628) have good stability. Sample RM-R152407 (code FG-6488) has good stability in acidic and neutral solutions, but poor stability in alkaline solutions. Attention should be paid to storage and detection processes. If you need stability data for the above three samples, welcome to consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号