Time:2024-05-20
01 Introduction
Carfilzomib is a specific and irreversible targeted proteasome inhibitor that can be used for the treatment of multiple myeloma. Today, we will share a summary of research on the stability of solutions containing specific impurities in anticancer drug Carfilzomib.
02 Experimental plan
In this experiment, our center conducted a solution stability study on four specific code impurities of Carfilzomib, RM-K161821 (code PR-019), RM-K161823 (code PR-059428), RM-K161825 (code PR-059462), and RM-K161840 (code PR-187), in accordance with the chromatographic conditions used under the "Related Substances" section of the "Import Registration Standard of Carfilzomib for Injection". The sample product numbers and structural formulas used are shown in Figure 1 and Figure 2:
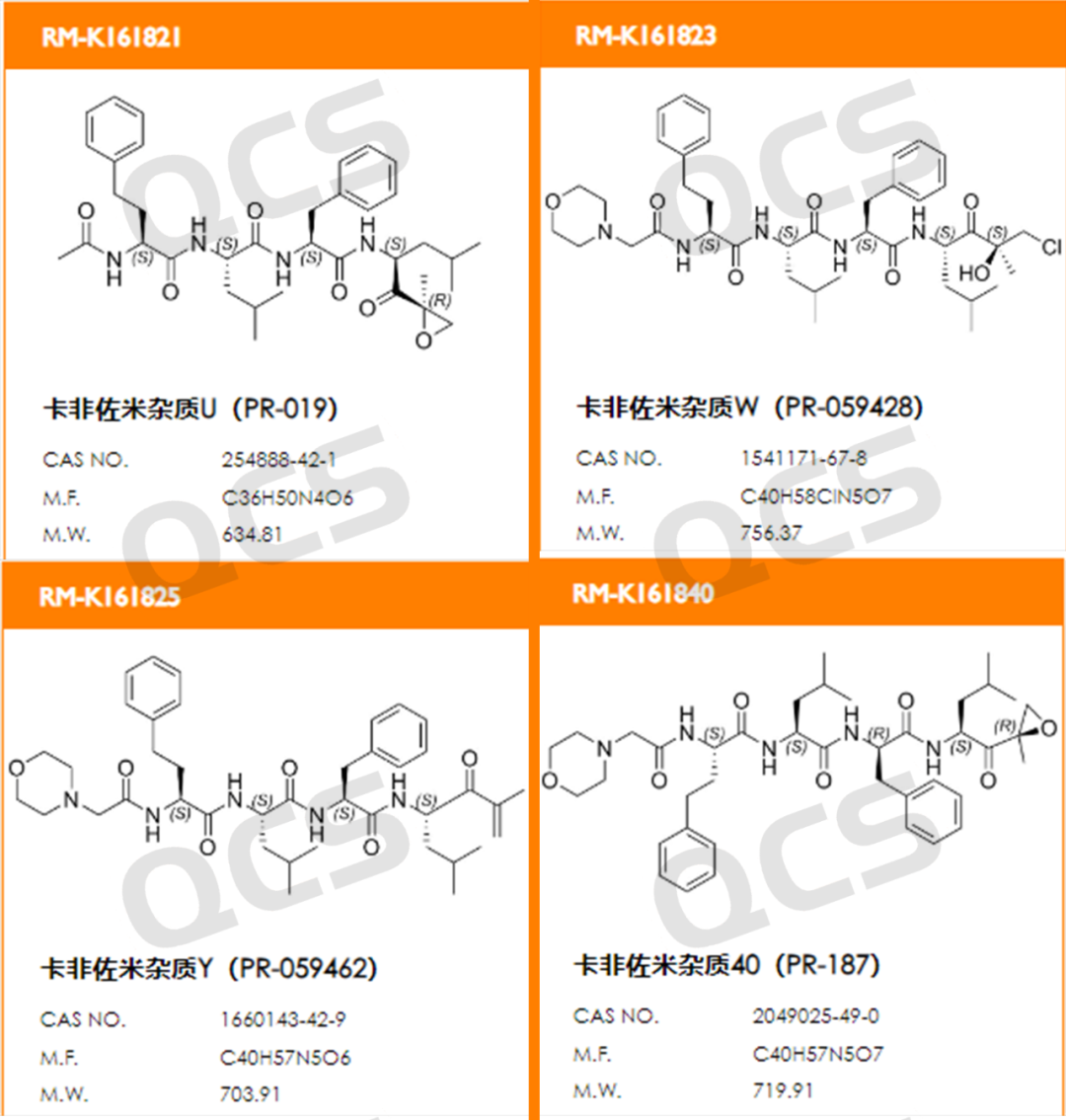
Figure 1: Product numbers and structural formulas of four impurities in Carfilzomib
(source: QCS Standard Material Research and Development Center)
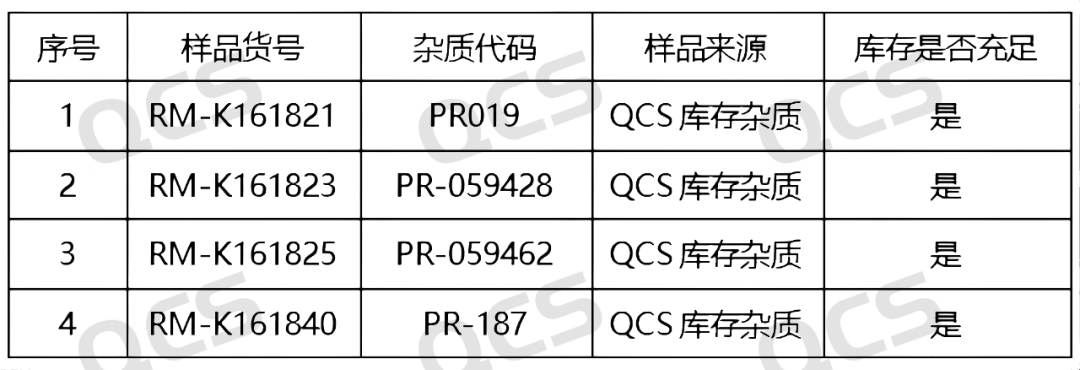
Figure 2: Correspondence between impurity codes and QCS item numbers included in the standard (source: QCS Standard Material Research and Development Center)
In this experiment, the experimenter took appropriate amounts of RM-K161821 (PR-019), RM-K161823 (PR-059428), RM-K161825 (PR-059462), and RM-K161840 (PR-187) each and placed them in acidic, neutral, and alkaline solutions, respectively. Samples were placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, and were injected and tested according to the chromatographic conditions used in the "Related Substances" section of the "Registration Standard for Injection of Carfilzomib". Observe the changes in the peak area of the main peak of the sample as the storage time increases, and use this as a basis to evaluate the solution stability of the sample.
03 Experiment conclusion
After testing, it was found that the main peak area of samples RM-K161821 (PR-019), RM-K161823 (PR-059428), RM-K161825 (PR-059462), and RM-K161840 (PR-187) did not change much during the 24-hour storage in acidic and neutral solutions, and the relative standard deviation was all less than 2.0%, it can be considered that these four samples are relatively stable after being stored in acidic and neutral solutions for 24 hours. However, when the sample is in an alkaline solution, varying degrees of degradation can be observed over time. The main peak area of the above four samples in acidic, neutral, and alkaline solutions varies with time as follows:
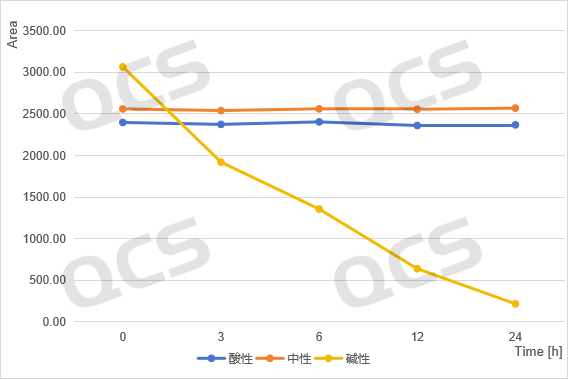
acidic, neutral, alkaline
Figure 3: Summary of Stability Data for RM-K161821 (Code PR-019) Solution
(Source: QCS Standard Material Research and Development Center)
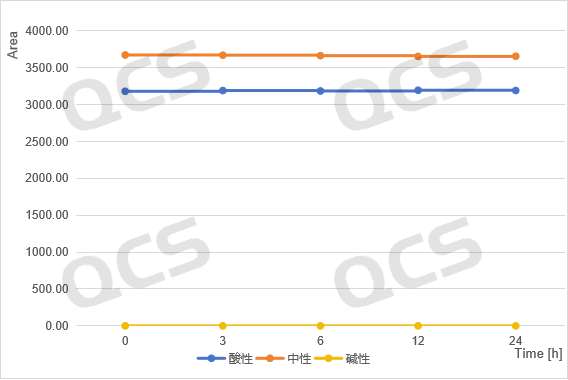
acidic, neutral, alkaline
Figure 4: Summary of Stability Data for RM-K161823 (Code PR-059428) Solution
(Source: QCS Reference Material Research and Development Center)
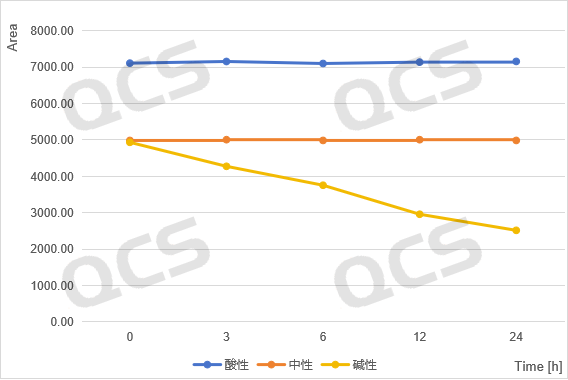
acidic, neutral, alkaline
Figure 5: Summary of Stability Data for RM-K161825 (Code PR-059462) Solution
(Source: QCS Reference Material Research and Development Center)
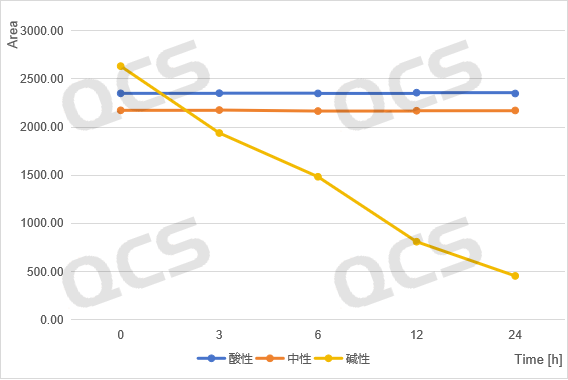
acidic, neutral, alkaline
Figure 6: Summary of Stability Data for RM-K161840 (Code PR-187) Solution
(Source: QCS Standard Material Research and Development Center)
From Figure 3-6, it can be seen that the main peak areas of impurities RM-K161821 (PR-019), RM-K161825 (PR-059462), and RM-K161840 (PR-187) did not change significantly during the 24 hour storage in acidic and neutral solutions. However, during the alkaline solution storage process, as the storage time prolonged, the area of the main peak decreases continuously, while the area of the impurity peak increases continuously, and the product will undergo degradation over time.
Although the experimental data of the above three products have preliminarily shown the intolerance of Carfilzomib impurity products to alkaline conditions, this problem mainly focuses on anomalies in quantitative data, which can be discovered through data comparison at different times. However, it is worth noting that product RM-K161823 (PR-059428) undergoes rapid degradation under alkaline conditions, which may lead to potential qualitative and quantitative errors in impurity research. This issue provides a more obvious warning for the rational use of this product.
Regarding the stability research data of product RM-K161823 (PR-059428) under alkaline conditions, if the 0-hour sample test spectrum is used as the standard, it can be observed that with the extension of storage time, the peak area of the main peak will change, and the rate of change is relatively slow. However, identification of its main peak revealed that the product RM-K161823 (PR-059428) would undergo complete degradation within a few minutes in alkaline solution. When the sample was placed in alkaline solution for 0.0 hours, the detection spectrum showed that the sample had completely deteriorated, and the retention time of the main peak had changed from 16.190 minutes to 18.588 minutes. Moreover, with the extension of the placement time, the sample would also undergo secondary degradation, indicating that the impurity PR-059428 is extremely unstable in alkaline solution and would completely deteriorate when exposed to alkali. Using alkaline solvent to treat the sample may result in incorrect experimental results, leading to incorrect qualitative and quantitative results.
The detection spectra of the above samples at different time points under alkaline solution conditions are shown in Figure 7-10:
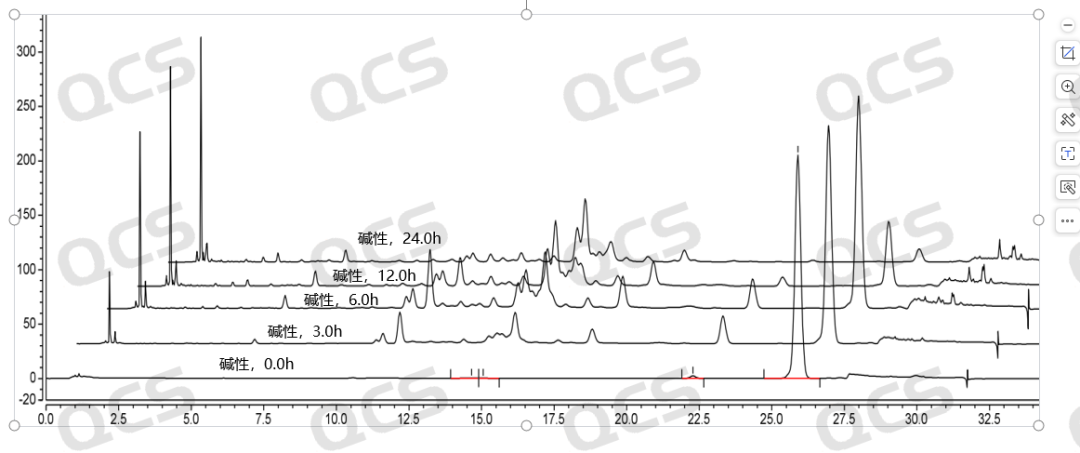
Figure 7: Stability Data graph of RM-K161821 (PR-019) in Alkaline Solution
(Source: QCS Standard Material Research and Development Center)
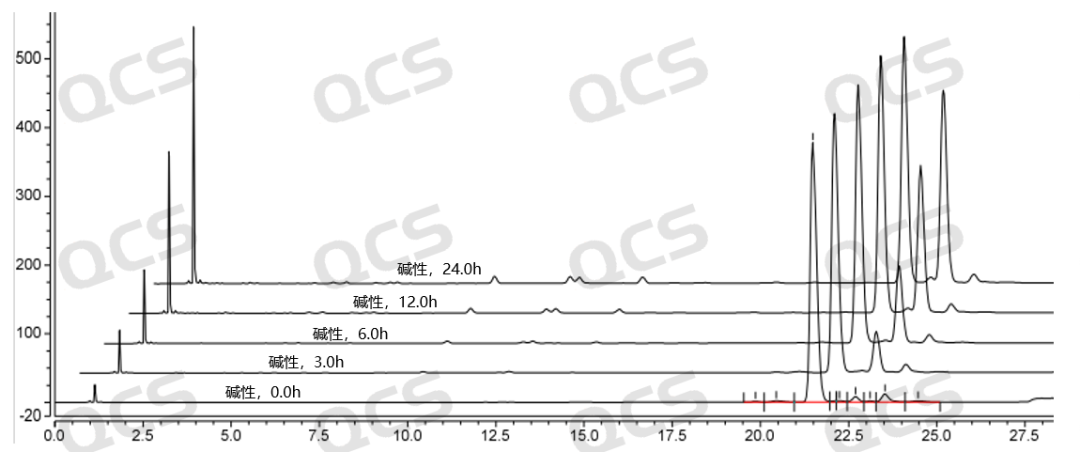
Figure 8: Stability data graph of RM-K161825 (PR-059462) in alkaline solution
(source: QCS Standard Material Research and Development Center)
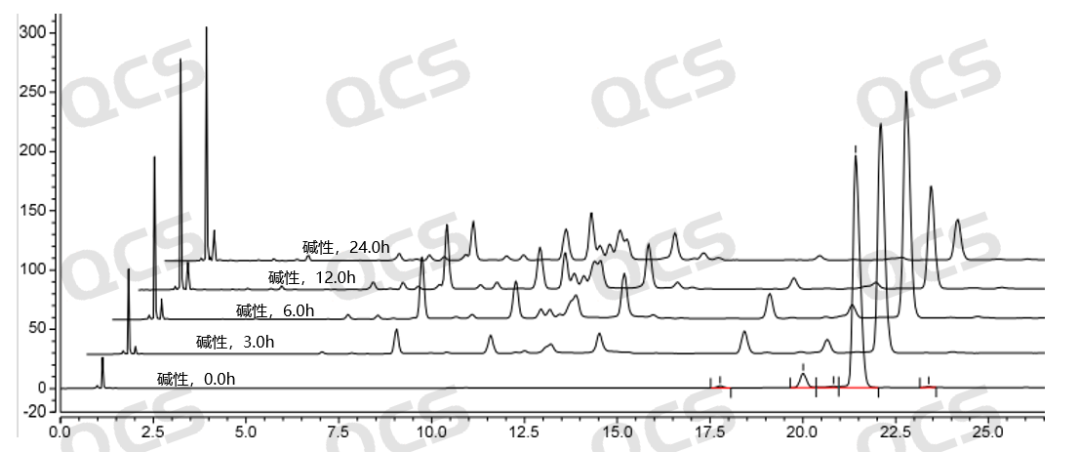
Figure 9: Stability data graph of RM-K161840 (PR-187) in alkaline solution
(source: QCS Standard Material Research and Development Center)
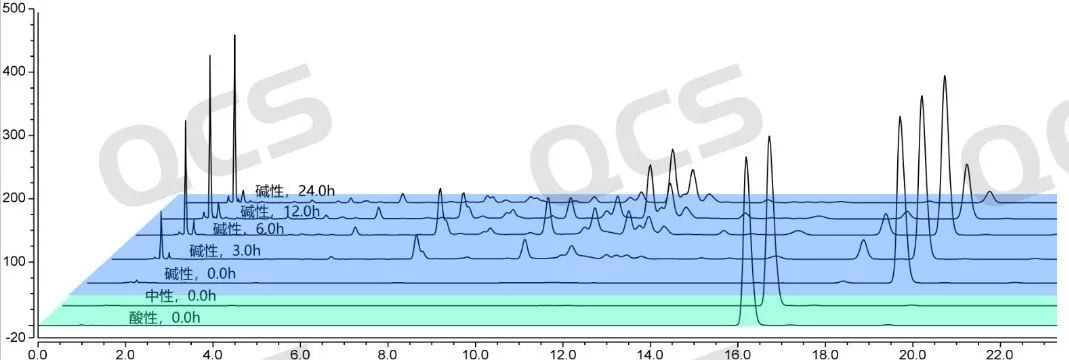
Figure 10: Stability data graph of PR-059428 in alkaline solution
(source: QCS Standard Material Research and Development Center)
04 summary
In summary, through this experiment, we found that the main peak areas of RM-K161821 (PR-019), RM-K161823 (PR-059428), RM-K161825 (PR-059462), and RM-K161840 (PR-187) impurities did not change significantly in acidic and neutral solutions, indicating that these four samples have good stability in acidic and neutral solutions; However, as the storage time increases in alkaline solution, the main peak area of these four samples will continuously decrease, indicating that these four samples are unstable in alkaline solution and will continuously degrade with the extension of storage time. Especially, the impurity RM-K161823 (PR-059428) will completely deteriorate during the preparation process with alkaline diluent, and secondary degradation will occur with the extension of storage time. So customers should try to avoid using alkaline solutions or alkaline conditions when detecting impurities such as Carfilzomib. If customers are interested in the stability research of Carfilzomib impurities, welcome to consult and request from our company.


01 Introduction
Carfilzomib is a specific and irreversible targeted proteasome inhibitor that can be used for the treatment of multiple myeloma. Today, we will share a summary of research on the stability of solutions containing specific impurities in anticancer drug Carfilzomib.
02 Experimental plan
In this experiment, our center conducted a solution stability study on four specific code impurities of Carfilzomib, RM-K161821 (code PR-019), RM-K161823 (code PR-059428), RM-K161825 (code PR-059462), and RM-K161840 (code PR-187), in accordance with the chromatographic conditions used under the "Related Substances" section of the "Import Registration Standard of Carfilzomib for Injection". The sample product numbers and structural formulas used are shown in Figure 1 and Figure 2:

Figure 1: Product numbers and structural formulas of four impurities in Carfilzomib
(source: QCS Standard Material Research and Development Center)

Figure 2: Correspondence between impurity codes and QCS item numbers included in the standard (source: QCS Standard Material Research and Development Center)
In this experiment, the experimenter took appropriate amounts of RM-K161821 (PR-019), RM-K161823 (PR-059428), RM-K161825 (PR-059462), and RM-K161840 (PR-187) each and placed them in acidic, neutral, and alkaline solutions, respectively. Samples were placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, and were injected and tested according to the chromatographic conditions used in the "Related Substances" section of the "Registration Standard for Injection of Carfilzomib". Observe the changes in the peak area of the main peak of the sample as the storage time increases, and use this as a basis to evaluate the solution stability of the sample.
03 Experiment conclusion
After testing, it was found that the main peak area of samples RM-K161821 (PR-019), RM-K161823 (PR-059428), RM-K161825 (PR-059462), and RM-K161840 (PR-187) did not change much during the 24-hour storage in acidic and neutral solutions, and the relative standard deviation was all less than 2.0%, it can be considered that these four samples are relatively stable after being stored in acidic and neutral solutions for 24 hours. However, when the sample is in an alkaline solution, varying degrees of degradation can be observed over time. The main peak area of the above four samples in acidic, neutral, and alkaline solutions varies with time as follows:

acidic, neutral, alkaline
Figure 3: Summary of Stability Data for RM-K161821 (Code PR-019) Solution
(Source: QCS Standard Material Research and Development Center)

acidic, neutral, alkaline
Figure 4: Summary of Stability Data for RM-K161823 (Code PR-059428) Solution
(Source: QCS Reference Material Research and Development Center)

acidic, neutral, alkaline
Figure 5: Summary of Stability Data for RM-K161825 (Code PR-059462) Solution
(Source: QCS Reference Material Research and Development Center)

acidic, neutral, alkaline
Figure 6: Summary of Stability Data for RM-K161840 (Code PR-187) Solution
(Source: QCS Standard Material Research and Development Center)
From Figure 3-6, it can be seen that the main peak areas of impurities RM-K161821 (PR-019), RM-K161825 (PR-059462), and RM-K161840 (PR-187) did not change significantly during the 24 hour storage in acidic and neutral solutions. However, during the alkaline solution storage process, as the storage time prolonged, the area of the main peak decreases continuously, while the area of the impurity peak increases continuously, and the product will undergo degradation over time.
Although the experimental data of the above three products have preliminarily shown the intolerance of Carfilzomib impurity products to alkaline conditions, this problem mainly focuses on anomalies in quantitative data, which can be discovered through data comparison at different times. However, it is worth noting that product RM-K161823 (PR-059428) undergoes rapid degradation under alkaline conditions, which may lead to potential qualitative and quantitative errors in impurity research. This issue provides a more obvious warning for the rational use of this product.
Regarding the stability research data of product RM-K161823 (PR-059428) under alkaline conditions, if the 0-hour sample test spectrum is used as the standard, it can be observed that with the extension of storage time, the peak area of the main peak will change, and the rate of change is relatively slow. However, identification of its main peak revealed that the product RM-K161823 (PR-059428) would undergo complete degradation within a few minutes in alkaline solution. When the sample was placed in alkaline solution for 0.0 hours, the detection spectrum showed that the sample had completely deteriorated, and the retention time of the main peak had changed from 16.190 minutes to 18.588 minutes. Moreover, with the extension of the placement time, the sample would also undergo secondary degradation, indicating that the impurity PR-059428 is extremely unstable in alkaline solution and would completely deteriorate when exposed to alkali. Using alkaline solvent to treat the sample may result in incorrect experimental results, leading to incorrect qualitative and quantitative results.
The detection spectra of the above samples at different time points under alkaline solution conditions are shown in Figure 7-10:

Figure 7: Stability Data graph of RM-K161821 (PR-019) in Alkaline Solution
(Source: QCS Standard Material Research and Development Center)

Figure 8: Stability data graph of RM-K161825 (PR-059462) in alkaline solution
(source: QCS Standard Material Research and Development Center)

Figure 9: Stability data graph of RM-K161840 (PR-187) in alkaline solution
(source: QCS Standard Material Research and Development Center)

Figure 10: Stability data graph of PR-059428 in alkaline solution
(source: QCS Standard Material Research and Development Center)
04 summary
In summary, through this experiment, we found that the main peak areas of RM-K161821 (PR-019), RM-K161823 (PR-059428), RM-K161825 (PR-059462), and RM-K161840 (PR-187) impurities did not change significantly in acidic and neutral solutions, indicating that these four samples have good stability in acidic and neutral solutions; However, as the storage time increases in alkaline solution, the main peak area of these four samples will continuously decrease, indicating that these four samples are unstable in alkaline solution and will continuously degrade with the extension of storage time. Especially, the impurity RM-K161823 (PR-059428) will completely deteriorate during the preparation process with alkaline diluent, and secondary degradation will occur with the extension of storage time. So customers should try to avoid using alkaline solutions or alkaline conditions when detecting impurities such as Carfilzomib. If customers are interested in the stability research of Carfilzomib impurities, welcome to consult and request from our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号