Time:2024-05-16
01 Introduction
Introduction: Today, we will share antifungal drugs-research on the stability of Amorolfine specific impurity solutions. Amorolfine can inhibit various steps of fungal cell wall ergosterol biosynthesis, interfere with the synthesis of fungal cell wall ergosterol, and lead to fungal death. Amorolfine has antibacterial activity against various pathogenic bacteria such as dermatophytes, Candida, dermatitis spore forming bacteria, Cryptococcus, capsule tissue cytoplasmic bacteria, Schenck sporotrichosis, etc. In addition, this product has good effects on vaginal candida albicans, onychomycosis, and various skin fungal diseases, but is ineffective against vaginal Gram positive bacterial infections and has no effect on organ fungal infections.
In this experiment, our center conducted a solution stability study on three specific impurities (Impurity A, B, E) of Amorolfine based on the chromatographic conditions used under the "Related Substances" section of the "Amorolfine hydrochloride" variety in the European Pharmacopoeia 11.0 edition. The sample product numbers and structural formulas used are shown in Figure 1 and Figure 2:
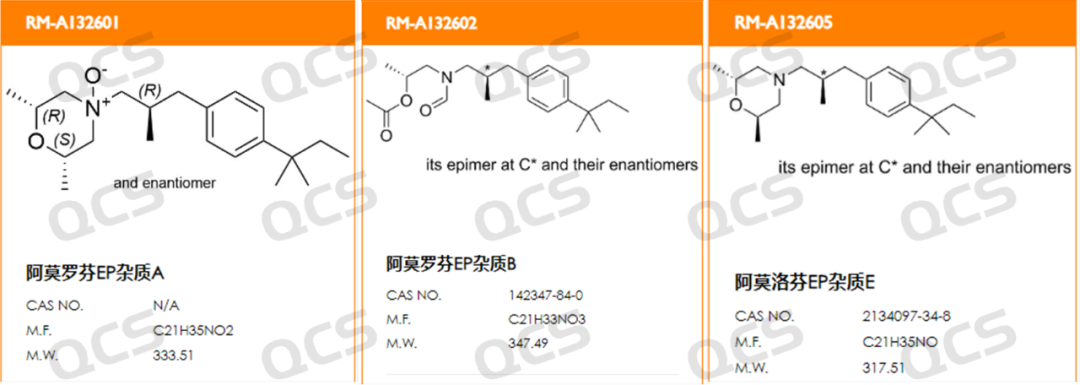
Figure 1: Structural formulas of impurities A, B, and E in Amorolfine EP (source: QCS official website: https://www.qcsrm.com/ )
02 Specific impurity research:
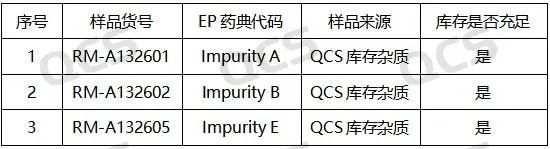
Figure 2: Correspondence between EP Standard Impurity Code and QCS Item Number (Source: QCS Standard Material R&D Center)
In this experiment, the experimenter took appropriate amounts of RM-A132601 (Impurity A), RM-A132602 (Impurity B), and RM-A132605 (Impurity E) each and placed them in solutions with pH=acidic, neutral, and alkaline. They were placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively, and then injected according to the chromatographic conditions used under the "Related substances" section of the "Amorolfine hydrochloride" variety item in the 11.0 version of the European Pharmacopoeia. The changes in the peak area of the main peak of the sample were observed as the sample was placed for an extended period of time. This was used as a basis for analysis. Determine the solution stability of the sample based on.
After testing, it was found that the main peak area of sample RM-A132605 did not change significant during 24 hours of storage in pH=acidic, neutral, and alkaline solutions, with a relative standard deviation of less than 2.0%.. Therefore, it can be considered that RM-A132605 is relatively stable during 24 hours of storage in pH=acidic, neutral, and alkaline solutions. The main peak area data of each detection point of the sample under various pH conditions are as follows:
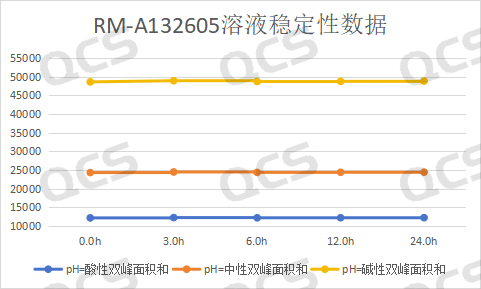
Figure 3: Line chart of solution stability data for impurity E in Amorolfine (source: QCS Standard Substance R&D Center)
Blue line - acidic, Orange Line - Neutral, Yellow line - alkaline
During the testing process, the experimenter found that the main peak area of sample RM-A132601 did not change significantly when placed in pH=acidic and neutral solutions for 24 hours, with a relative standard deviation of less than 2.0%. This indicates that sample RM-A132601 is relatively stable when placed in acidic and neutral solutions for 24 hours. However, during the process of placing the sample in pH=alkaline solutions for 24 hours, the main peak area continuously decreases and the impurity peak area continuously increases with the extension of the placement time, indicating that sample RM-A132601 is unstable in alkaline solutions and will degrade with the extension of time. The main peak of this sample at each detection point under various pH conditions The area data is as follows:
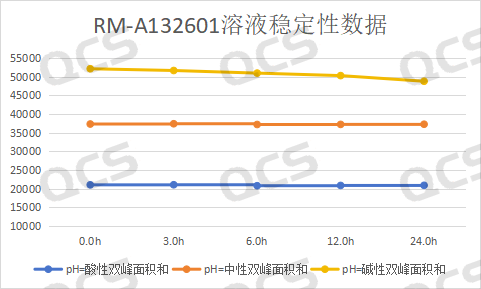
Figure 4: Line chart of stability data for Amorolfine Impurity A solution (source: QCS Standard Substance R&D Center)
Blue line - acidic, Orange Line - Neutral, Yellow line - alkaline
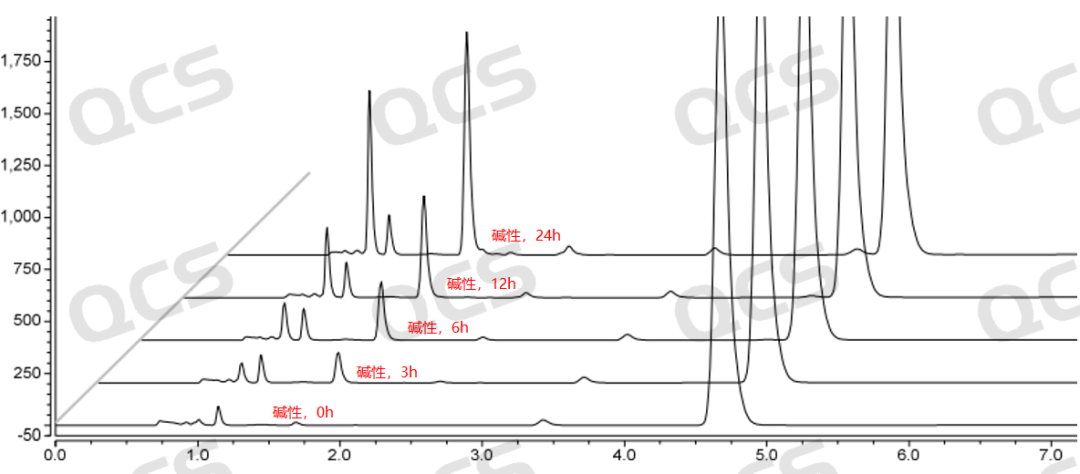
Figure 5: Stability chromatogram of Amorolfine impurity A in alkaline solution (source: QCS Standard Material R&D Center)
After testing, the experimenter found that the area of the main peak of sample RM-A132602 did not change significantly when placed in pH=acidic and neutral solutions for 24 hours, with a relative standard deviation of less than 2.0%. This indicates that sample RM-A132602 is relatively stable when placed in acidic and neutral solutions for 24 hours. However, the sample is extremely unstable in pH=alkaline solutions. Compared with the detection spectrum of the detection point placed in pH=neutral solutions for 0.0h, the detection spectrum of the main peak area of the sample is significantly reduced when placed in alkaline solutions for 0.0h, indicating that the sample has partially degraded. The detection spectrum of the detection point placed in alkaline solutions for 3.0h shows that the sample has undergone partial degradation. It is shown that the main peak area of the sample is 0, indicating that the sample has been completely degraded. Based on this, it is speculated that sample RM-A132602 is very unstable in alkaline solution and will degrade rapidly in alkaline solution, special attention should be paid when preparing the sample. The main peak area data at each detection point of the sample under various pH conditions are as follows:
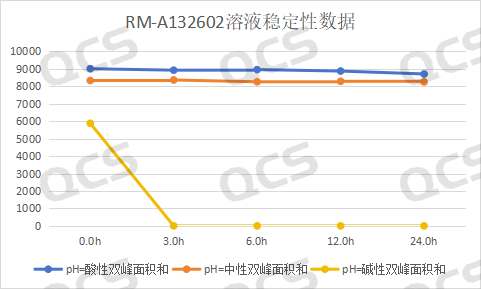
Figure 6: Line chart of solution stability data for impurity B in Amorolfine (source: QCS Standard Substance R&D Center)
Blue line - acidic, Orange Line - Neutral, Yellow line - alkaline
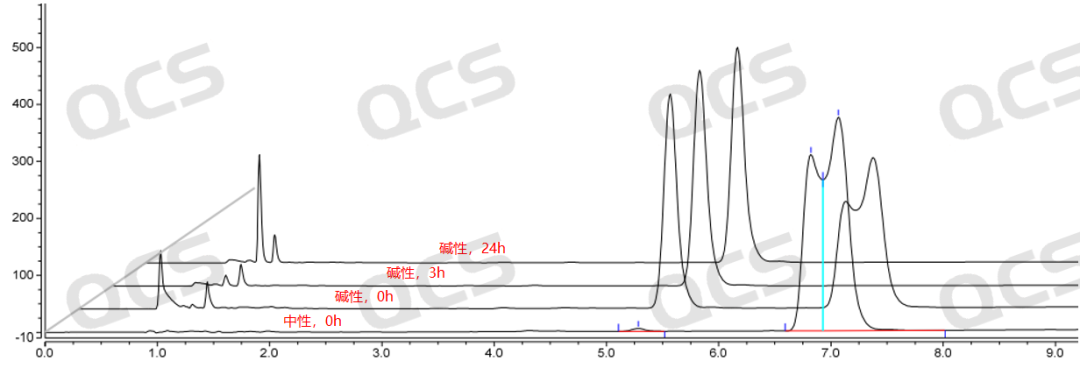
Figure 7: Stability chromatogram of Amorolfine impurity B in alkaline solution (source: QCS Standard Material R&D Center)
In summary, through this experiment, we found that the impurity E of amoprofen is relatively stable in acidic, alkaline, and neutral solutions; Amoprofen impurity A has good stability in acidic and neutral solutions, but it is not very stable in alkaline solutions and will degrade over time. Therefore, customers should avoid using alkaline diluents when testing this sample. If it is necessary to prepare the sample with alkaline diluents, once prepared and then tested immediately, and the sample cannot be stored together with alkaline samples for a long time; Impurity B of Amorolfine is similar to Impurity A and has good stability in acidic and neutral solutions. However, Impurity B is extremely unstable in alkaline solutions, and even undergoes degradation during the sample preparation process. Therefore, Impurity B must avoid contact with alkali, otherwise it is difficult to obtain ideal experimental results. We hope our research data can be helpful for your experiment. If customers need stability data for these three samples, they can consult our company for assistance.
A small question: Impurity B rapidly degrades under alkaline conditions, and the degradation product generated is relatively stable during the 24-hour experimental process, so what is this degradation product? Welcome everyone to provide your own answers. The results will be announced in the next issue. Those who answer correctly will receive small gifts:)

01 Introduction
Introduction: Today, we will share antifungal drugs-research on the stability of Amorolfine specific impurity solutions. Amorolfine can inhibit various steps of fungal cell wall ergosterol biosynthesis, interfere with the synthesis of fungal cell wall ergosterol, and lead to fungal death. Amorolfine has antibacterial activity against various pathogenic bacteria such as dermatophytes, Candida, dermatitis spore forming bacteria, Cryptococcus, capsule tissue cytoplasmic bacteria, Schenck sporotrichosis, etc. In addition, this product has good effects on vaginal candida albicans, onychomycosis, and various skin fungal diseases, but is ineffective against vaginal Gram positive bacterial infections and has no effect on organ fungal infections.
In this experiment, our center conducted a solution stability study on three specific impurities (Impurity A, B, E) of Amorolfine based on the chromatographic conditions used under the "Related Substances" section of the "Amorolfine hydrochloride" variety in the European Pharmacopoeia 11.0 edition. The sample product numbers and structural formulas used are shown in Figure 1 and Figure 2:

Figure 1: Structural formulas of impurities A, B, and E in Amorolfine EP (source: QCS official website: https://www.qcsrm.com/ )
02 Specific impurity research:

Figure 2: Correspondence between EP Standard Impurity Code and QCS Item Number (Source: QCS Standard Material R&D Center)
In this experiment, the experimenter took appropriate amounts of RM-A132601 (Impurity A), RM-A132602 (Impurity B), and RM-A132605 (Impurity E) each and placed them in solutions with pH=acidic, neutral, and alkaline. They were placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively, and then injected according to the chromatographic conditions used under the "Related substances" section of the "Amorolfine hydrochloride" variety item in the 11.0 version of the European Pharmacopoeia. The changes in the peak area of the main peak of the sample were observed as the sample was placed for an extended period of time. This was used as a basis for analysis. Determine the solution stability of the sample based on.
After testing, it was found that the main peak area of sample RM-A132605 did not change significant during 24 hours of storage in pH=acidic, neutral, and alkaline solutions, with a relative standard deviation of less than 2.0%.. Therefore, it can be considered that RM-A132605 is relatively stable during 24 hours of storage in pH=acidic, neutral, and alkaline solutions. The main peak area data of each detection point of the sample under various pH conditions are as follows:

Figure 3: Line chart of solution stability data for impurity E in Amorolfine (source: QCS Standard Substance R&D Center)
Blue line - acidic, Orange Line - Neutral, Yellow line - alkaline
During the testing process, the experimenter found that the main peak area of sample RM-A132601 did not change significantly when placed in pH=acidic and neutral solutions for 24 hours, with a relative standard deviation of less than 2.0%. This indicates that sample RM-A132601 is relatively stable when placed in acidic and neutral solutions for 24 hours. However, during the process of placing the sample in pH=alkaline solutions for 24 hours, the main peak area continuously decreases and the impurity peak area continuously increases with the extension of the placement time, indicating that sample RM-A132601 is unstable in alkaline solutions and will degrade with the extension of time. The main peak of this sample at each detection point under various pH conditions The area data is as follows:

Figure 4: Line chart of stability data for Amorolfine Impurity A solution (source: QCS Standard Substance R&D Center)
Blue line - acidic, Orange Line - Neutral, Yellow line - alkaline

Figure 5: Stability chromatogram of Amorolfine impurity A in alkaline solution (source: QCS Standard Material R&D Center)
After testing, the experimenter found that the area of the main peak of sample RM-A132602 did not change significantly when placed in pH=acidic and neutral solutions for 24 hours, with a relative standard deviation of less than 2.0%. This indicates that sample RM-A132602 is relatively stable when placed in acidic and neutral solutions for 24 hours. However, the sample is extremely unstable in pH=alkaline solutions. Compared with the detection spectrum of the detection point placed in pH=neutral solutions for 0.0h, the detection spectrum of the main peak area of the sample is significantly reduced when placed in alkaline solutions for 0.0h, indicating that the sample has partially degraded. The detection spectrum of the detection point placed in alkaline solutions for 3.0h shows that the sample has undergone partial degradation. It is shown that the main peak area of the sample is 0, indicating that the sample has been completely degraded. Based on this, it is speculated that sample RM-A132602 is very unstable in alkaline solution and will degrade rapidly in alkaline solution, special attention should be paid when preparing the sample. The main peak area data at each detection point of the sample under various pH conditions are as follows:

Figure 6: Line chart of solution stability data for impurity B in Amorolfine (source: QCS Standard Substance R&D Center)
Blue line - acidic, Orange Line - Neutral, Yellow line - alkaline

Figure 7: Stability chromatogram of Amorolfine impurity B in alkaline solution (source: QCS Standard Material R&D Center)
In summary, through this experiment, we found that the impurity E of amoprofen is relatively stable in acidic, alkaline, and neutral solutions; Amoprofen impurity A has good stability in acidic and neutral solutions, but it is not very stable in alkaline solutions and will degrade over time. Therefore, customers should avoid using alkaline diluents when testing this sample. If it is necessary to prepare the sample with alkaline diluents, once prepared and then tested immediately, and the sample cannot be stored together with alkaline samples for a long time; Impurity B of Amorolfine is similar to Impurity A and has good stability in acidic and neutral solutions. However, Impurity B is extremely unstable in alkaline solutions, and even undergoes degradation during the sample preparation process. Therefore, Impurity B must avoid contact with alkali, otherwise it is difficult to obtain ideal experimental results. We hope our research data can be helpful for your experiment. If customers need stability data for these three samples, they can consult our company for assistance.
A small question: Impurity B rapidly degrades under alkaline conditions, and the degradation product generated is relatively stable during the 24-hour experimental process, so what is this degradation product? Welcome everyone to provide your own answers. The results will be announced in the next issue. Those who answer correctly will receive small gifts:)

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号