Time:2024-05-16
01 Introduction
Today we would like to share the adrenocortical hormone drug-dapagliflozin specific impurity solution stability research sharing. Dapagliflozin is a competitive glucose co-transporter 2 (SGLT2) inhibitor that can lead to the release of glucose in urine. Excretion, used in the study of diabetes mellitus (DM).
In this experiment, our laboratory analyzed the four specific impurities of dapagliflozin with reference to the chromatographic conditions used under the "related substances" in the import registration standards for "dapagliflozin" issued by the State Food and Drug Administration. For solution stability study, the catalogue numbers and structural formulas used are as follows, Figure 1 and Figure 2.
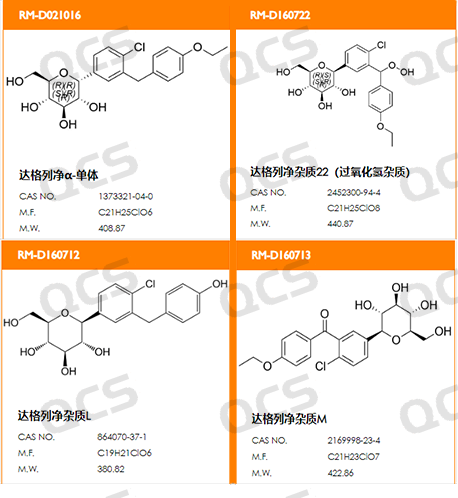
Figure 1: Impurity catalogue numbers and structural formulas used in this study
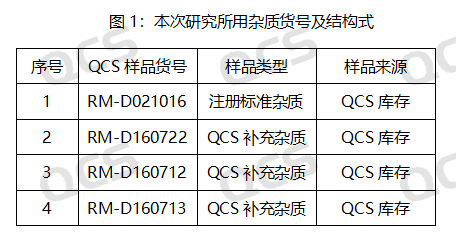
Figure 2: Correspondence diagram between the impurity codes included in APIs, standards and the impurity catalogue numbers used in this study
02 Specific impurity research
In this experiment, our experimenters took appropriate amounts of each of the following four impurities: RM-D021016 (dapagliflozin alpha isomer), RM-D160722 (empagliflozin peroxide), RM-D160712 ( deethyldapagliflozin) and RM-D160713 (code BMS-801575). Then place them in solutions with pH = A, B, and C, and inject samples for detection at around 0, 1, 2, 4, 8, 12, and 24 respectively. We found that the peak areas of the main peaks of the three samples RM-D021016, RM-D160712 and RM-D160713 did not change much when they were placed in solutions with pH=A, B and C for 24 hours. It can be considered that RM-D021016, RM-D160712 and RM-D160713, these three samples are relatively stable when placed in solutions with pH=A, B, and C for 24 hours. The peak area data of the main peaks of each detection point of these three samples under various pH conditions are as follows.
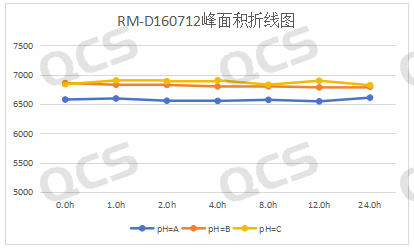
Figure 3: Summary line chart of solution stability data for sample RM-D160712
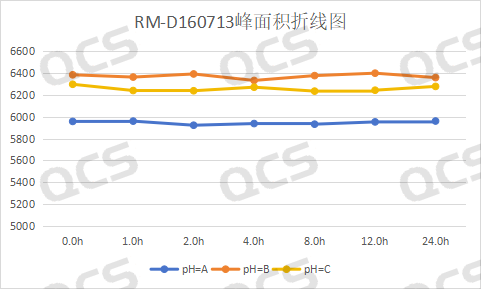
Figure 4: Summary line chart of solution stability data for sample RM-D160713

Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-D021016
03 Research on Peroxide Impurities
In this study, we found that the impurity of Dapagliflozin peroxide RM-D160722, which was the focus of observation, exhibited bimodal peaks under the chromatographic conditions used in the "Related Substances" section of the import registration standard of Dapagliflozin. So in this study, we developed a method to detect the molecular weight of these two chromatographic peaks on LCMS and found that the molecular weight of the MS peak corresponding to the two chromatographic peaks was the same. Therefore, the reason why the bimodal peaks of Dapagliflozin peroxide RM-D160722 under the chromatographic conditions used in the "Related Substances" section of the import registration standard of Dapagliflozin should be caused by the isomerism of chiral carbon atoms in the sample molecules.
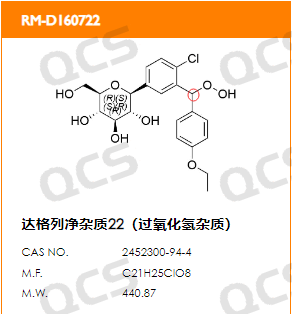
Figure 6: Schematic diagram of chiral carbon atoms in Dapagliflozin peroxide impurities
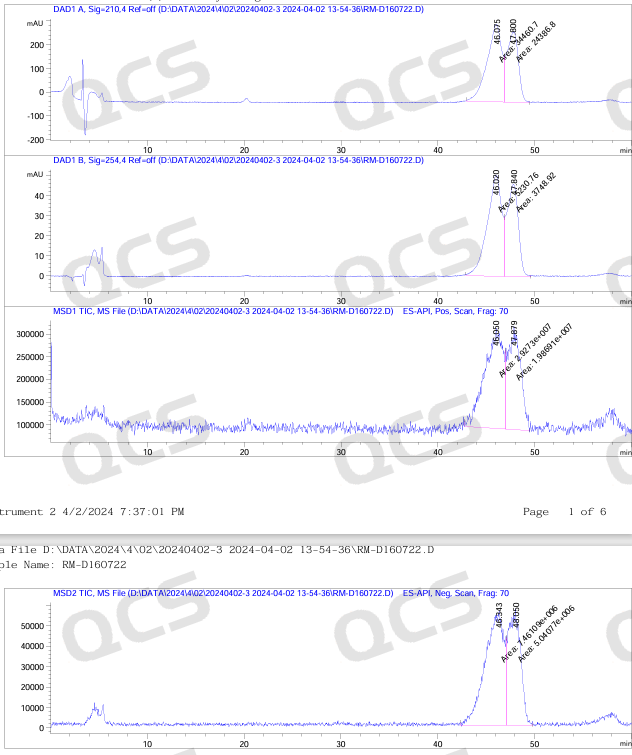
Figure 7: LCMS spectrum of impurities in Dapagliflozin peroxide
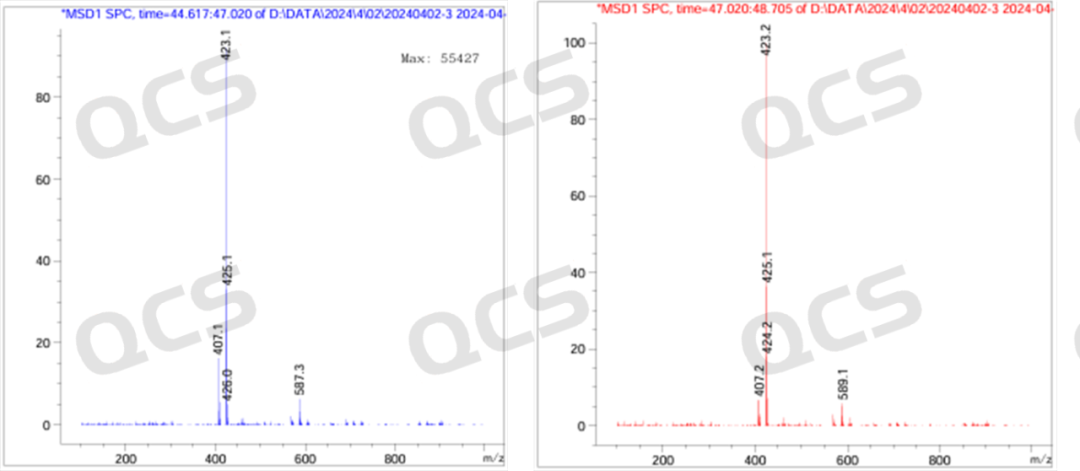
Figure 8: MS molecular weight diagram of Dapagliflozin peroxide impurities (positive ions)
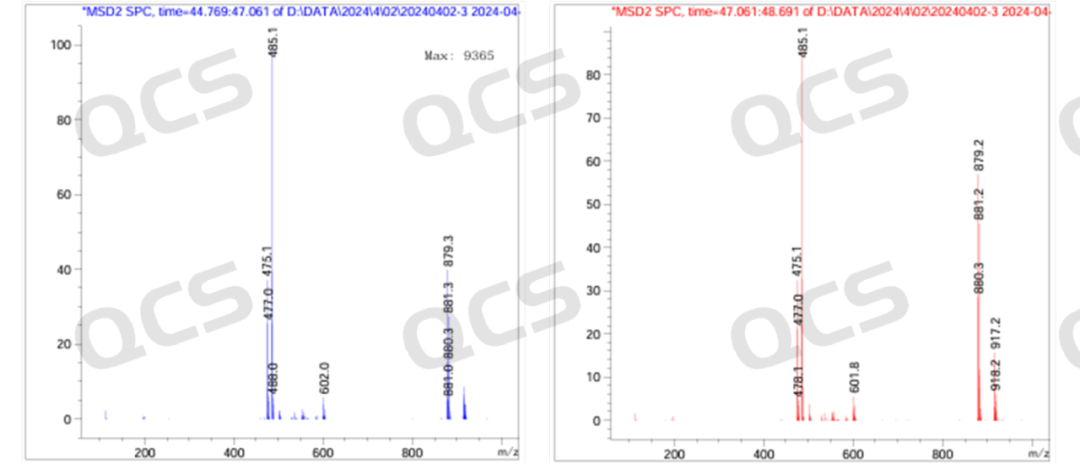
Figure 9: MS molecular weight diagram of Dapagliflozin peroxide impurities (negative ions)
From Figures 8 and 9, it can be seen that in the positive ion mode, both chromatographic peaks exhibit molecular weights of [M-OH], while in the negative ion mode, both chromatographic peaks exhibit molecular weights of [M+HCOOH]. Therefore, the LC-MS data can verify the previous idea.
In the subsequent stability testing process, we found that the areas of both chromatographic peaks of RM-D160722, a Dapagliflozin peroxide, degraded to varying degrees when placed in pH=A, B, and C solutions for 24 hours. Therefore, when the solution was placed for N days and injected again, it was found that the samples exhibited significant degradation, with the samples under pH=A and pH=C conditions showing significant degradation. The data summary line graph is shown below.
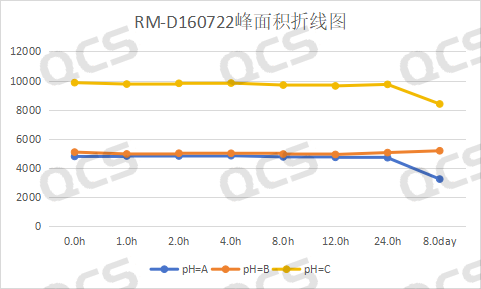
Figure 10: Stability data of Dapagliflozin peroxide in pH=A, B, C solutions
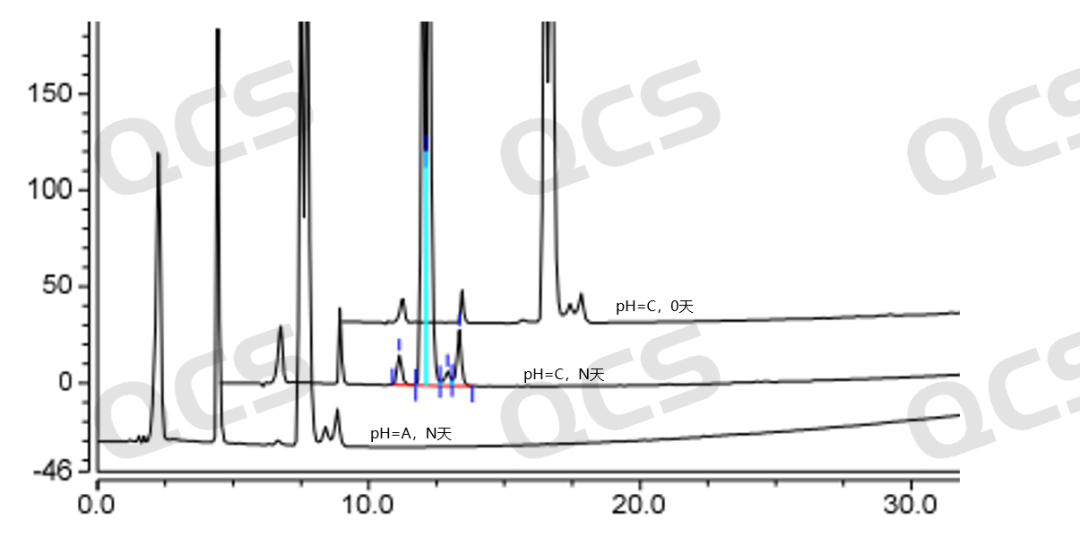
Figure 11: 3D plot of stability data of Dapagliflozin peroxide after being placed in different pH solutions for different times
04 Summary
From the above data, we can see that dapagliflozin peroxide RM-D160722 will degrade to varying degrees when placed in solutions with pH=A, B, and C for N days, especially at pH=A and pH. The degradation is obvious in the solution of =C, so when using this sample, customers should avoid using related acid and alkaline solutions to prepare the sample, try to prepare it immediately and test it, and pay attention to prevent degradation during storage.
In addition, our laboratory has also conducted solution stability experiments on dapagliflozin peroxide at different temperatures. If you have any needs for the stability content of this sample, please consult our company.
01 Introduction
Today we would like to share the adrenocortical hormone drug-dapagliflozin specific impurity solution stability research sharing. Dapagliflozin is a competitive glucose co-transporter 2 (SGLT2) inhibitor that can lead to the release of glucose in urine. Excretion, used in the study of diabetes mellitus (DM).
In this experiment, our laboratory analyzed the four specific impurities of dapagliflozin with reference to the chromatographic conditions used under the "related substances" in the import registration standards for "dapagliflozin" issued by the State Food and Drug Administration. For solution stability study, the catalogue numbers and structural formulas used are as follows, Figure 1 and Figure 2.

Figure 1: Impurity catalogue numbers and structural formulas used in this study

Figure 2: Correspondence diagram between the impurity codes included in APIs, standards and the impurity catalogue numbers used in this study
02 Specific impurity research
In this experiment, our experimenters took appropriate amounts of each of the following four impurities: RM-D021016 (dapagliflozin alpha isomer), RM-D160722 (empagliflozin peroxide), RM-D160712 ( deethyldapagliflozin) and RM-D160713 (code BMS-801575). Then place them in solutions with pH = A, B, and C, and inject samples for detection at around 0, 1, 2, 4, 8, 12, and 24 respectively. We found that the peak areas of the main peaks of the three samples RM-D021016, RM-D160712 and RM-D160713 did not change much when they were placed in solutions with pH=A, B and C for 24 hours. It can be considered that RM-D021016, RM-D160712 and RM-D160713, these three samples are relatively stable when placed in solutions with pH=A, B, and C for 24 hours. The peak area data of the main peaks of each detection point of these three samples under various pH conditions are as follows.

Figure 3: Summary line chart of solution stability data for sample RM-D160712

Figure 4: Summary line chart of solution stability data for sample RM-D160713

Figure 5: Summary Line Chart of Solution Stability Data for Sample RM-D021016
03 Research on Peroxide Impurities
In this study, we found that the impurity of Dapagliflozin peroxide RM-D160722, which was the focus of observation, exhibited bimodal peaks under the chromatographic conditions used in the "Related Substances" section of the import registration standard of Dapagliflozin. So in this study, we developed a method to detect the molecular weight of these two chromatographic peaks on LCMS and found that the molecular weight of the MS peak corresponding to the two chromatographic peaks was the same. Therefore, the reason why the bimodal peaks of Dapagliflozin peroxide RM-D160722 under the chromatographic conditions used in the "Related Substances" section of the import registration standard of Dapagliflozin should be caused by the isomerism of chiral carbon atoms in the sample molecules.

Figure 6: Schematic diagram of chiral carbon atoms in Dapagliflozin peroxide impurities

Figure 7: LCMS spectrum of impurities in Dapagliflozin peroxide

Figure 8: MS molecular weight diagram of Dapagliflozin peroxide impurities (positive ions)

Figure 9: MS molecular weight diagram of Dapagliflozin peroxide impurities (negative ions)
From Figures 8 and 9, it can be seen that in the positive ion mode, both chromatographic peaks exhibit molecular weights of [M-OH], while in the negative ion mode, both chromatographic peaks exhibit molecular weights of [M+HCOOH]. Therefore, the LC-MS data can verify the previous idea.
In the subsequent stability testing process, we found that the areas of both chromatographic peaks of RM-D160722, a Dapagliflozin peroxide, degraded to varying degrees when placed in pH=A, B, and C solutions for 24 hours. Therefore, when the solution was placed for N days and injected again, it was found that the samples exhibited significant degradation, with the samples under pH=A and pH=C conditions showing significant degradation. The data summary line graph is shown below.

Figure 10: Stability data of Dapagliflozin peroxide in pH=A, B, C solutions

Figure 11: 3D plot of stability data of Dapagliflozin peroxide after being placed in different pH solutions for different times
04 Summary
From the above data, we can see that dapagliflozin peroxide RM-D160722 will degrade to varying degrees when placed in solutions with pH=A, B, and C for N days, especially at pH=A and pH. The degradation is obvious in the solution of =C, so when using this sample, customers should avoid using related acid and alkaline solutions to prepare the sample, try to prepare it immediately and test it, and pay attention to prevent degradation during storage.
In addition, our laboratory has also conducted solution stability experiments on dapagliflozin peroxide at different temperatures. If you have any needs for the stability content of this sample, please consult our company.
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号