Time:2024-01-14
01 Introduction
Today, we will share the research on impurities related to non steroidal selective mineralocorticoid receptor antagonists -
Finarenone. Currently, there are 2 registration approval numbers for non phenanthrone preparations and raw materials in China, including 2 formulation approval numbers and no raw material approval number.
02 Introduction to Finarenone
Finelinone is a non steroidal selective mineralocorticoid receptor antagonist. In preclinical studies, it has been shown that Finelinone can block the harmful effects caused by excessive activation of the mineralocorticoid receptor, thus it can be used to treat chronic kidney disease related to type 2 diabetes, and reduce the risk of continuous decline in the estimated glomerular filtration rate (eGFR) and end-stage renal disease.
At present, the QCS official website has collected a total of 122 impurities of non noridone (scan the QR code at the end of the article to view the list of all impurities).Our laboratory has conducted liquid chromatography localization research on some popular impurities in the company's inventory using the HPLC chromatographic conditions used in the import registration standard of "Non noridone Tablets" (standard number JX20220072). The impurity code of the tablet and the structure information of the customer's key research impurities are shown in Figure 1.
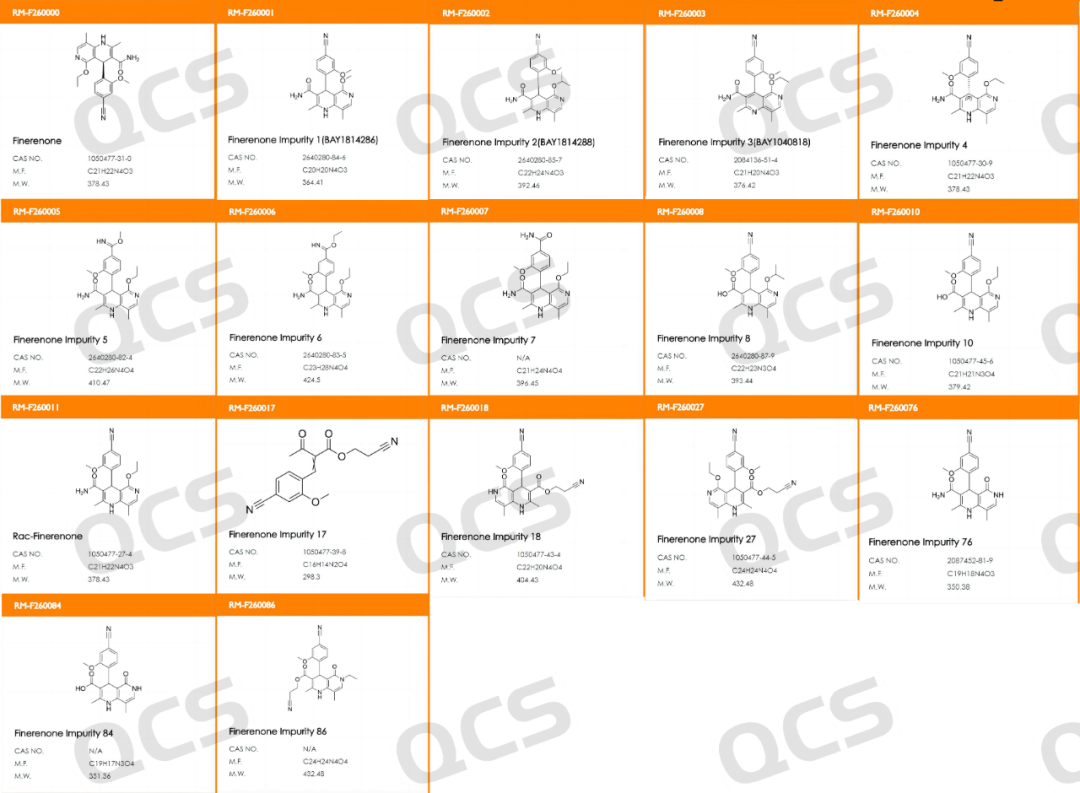
Figure 1: List of impurities focused on research by nonelidone-related customers
03 Typical chromatograms and measured results of related impurities
Referring to the relevant import registration standards, two detection conditions are provided for the substance inspection items of non noridone tablets. The relevant information and typical chromatogram are shown in Figure 2. In this study, the QCS R&D center referred to the HPLC detection conditions and made appropriate adjustments to the relevant chromatographic conditions. The HPLC conditions and chromatogram results used in this study are shown in Figure 3.
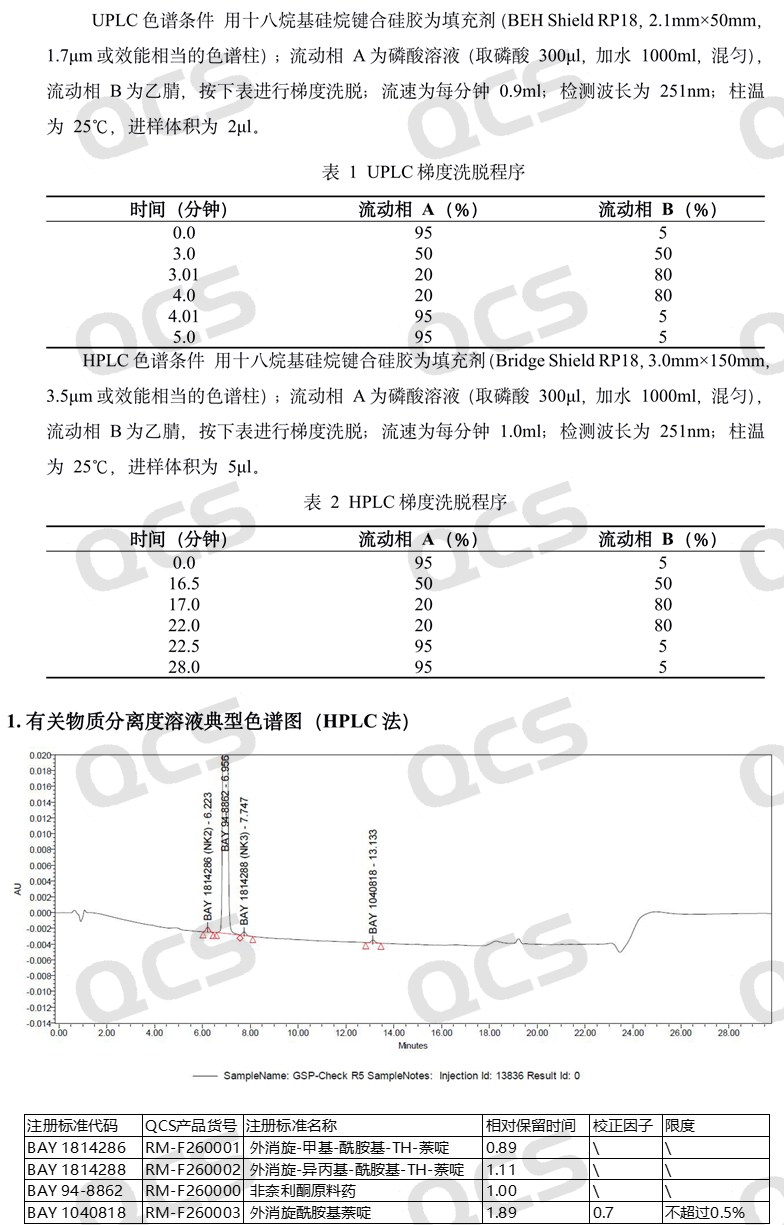
Figure 2: Typical chromatogram of mixed injection of Finarenoneraw material and impurities
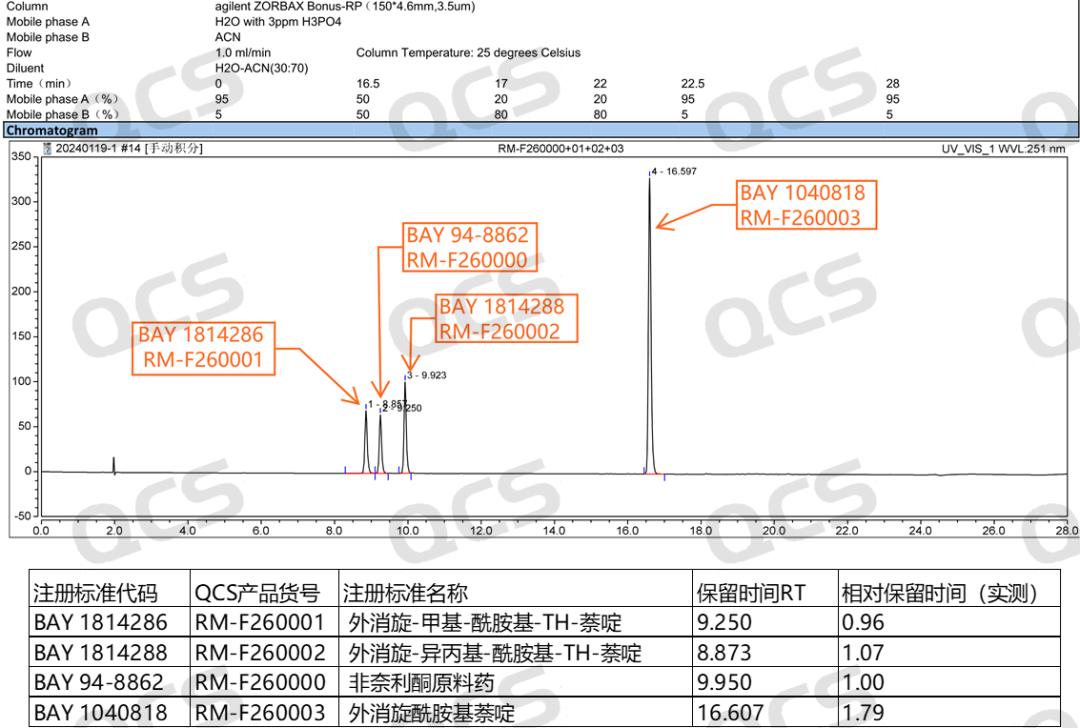
Figure 3: Typical results of QCS R&D center's measured mixed injection of non noridone raw materials and impurities
Although the stationary phase of the chromatography column in this study was not exactly the same as the standard, the three impurity codes BAY1814286, BAY1040818, and BAY1814288 included in the import registration standard for "Fenerlidone Tablets" (standard number JX20220072) were used as the judgment basis. In the actual liquid phase test, the elution order was consistent with the relevant standards, and the measured relative retention time was basically consistent with the data in the standard. It can be considered that the chromatographic results of the import registration standard were basically reproduced in this study.
Liquid phase results of 04 non-standard impurities
Using this liquid chromatography condition, the QCS R&D center conducted tests on multiple impurities that were not included in the standard but actually needed to be studied under the same conditions, in order to provide researchers with richer research results. In this study, the QCS R&D center collected a total of 10 impurity products in stock and conducted a series of studies using the same liquid chromatography conditions. The relative retention time and other data of all impurity products were calculated (as shown in Figure 4).
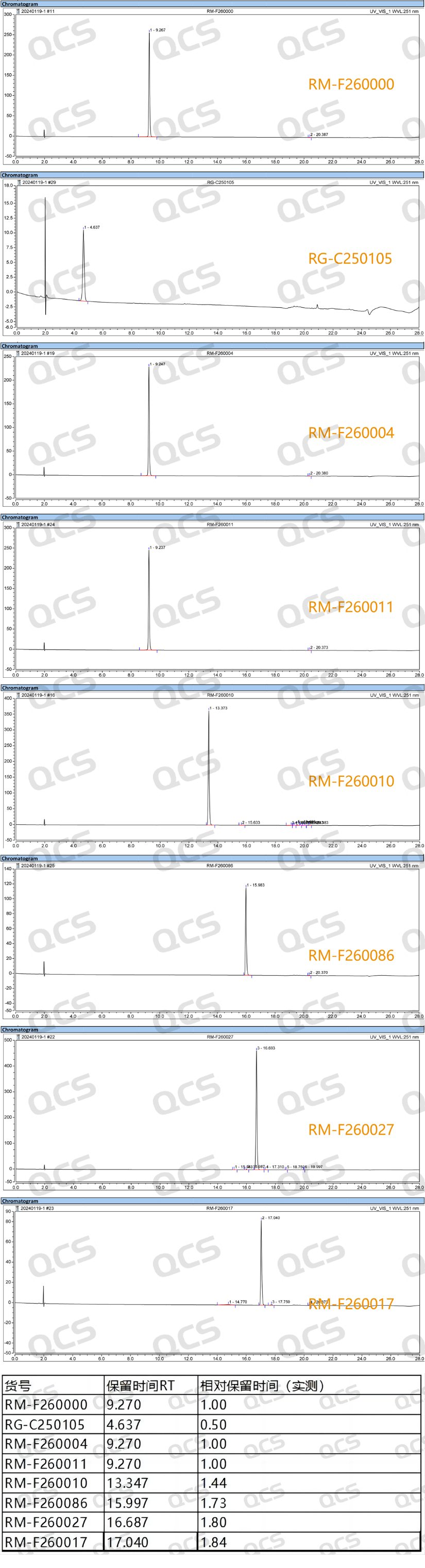
Figure 4: Localization spectra and data summary of 7 non registered
standard impurities under the same liquid chromatography conditions
The focus of the relevant import registration standard documents referred to this time is on the study of process impurities and degradation impurities, and no research has been conducted on chiral impurities. Therefore, the previous text lacks the ability to distinguish chiral impurities, especially those related to enantiomers.
From the data in Figure 4, it can be seen that under this chromatographic condition, when injecting API and multiple impurities simultaneously, three impurity products have the same retention time, namely RM-F260000, RM-F260004, and RM-F260011 (structural formula shown in Figure 5). The above three enantiomeric products cannot be distinguished under this condition.
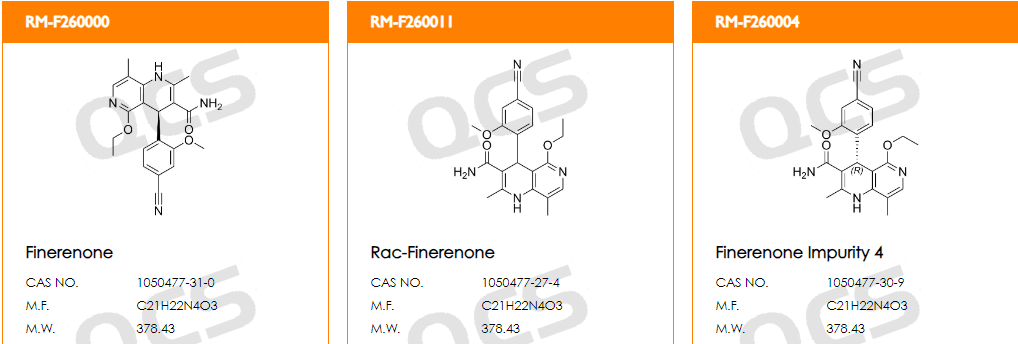
Figure 5: Structural information of RM-F260000&04&11
05 Analysis Method for Chiral Impurities of Non Nelitazone
However, for drug molecules with chiral centers, the study of their chiral impurities is inevitably an unavoidable issue. The catalogue numbers provided by the QCS R&D center are RM-F260000, RM-F260004, and RM-F260011, corresponding to (S) - Non Naridone standard (API, optically pure), (R) - Non Naridone standard (API enantiomer, optically pure), and racemic Non Naridone standard (racemic), respectively. The above three products can help researchers use them as controls in chiral development and detection work.
This article will not elaborate on the development of chiral analysis methods. Instead, we will share relevant information on the conditions of the corresponding isomer analysis methods for non naridone with researchers for reference (see Figure 6, source: Production process information table for non naridone tablets (JXHS210017-8)).
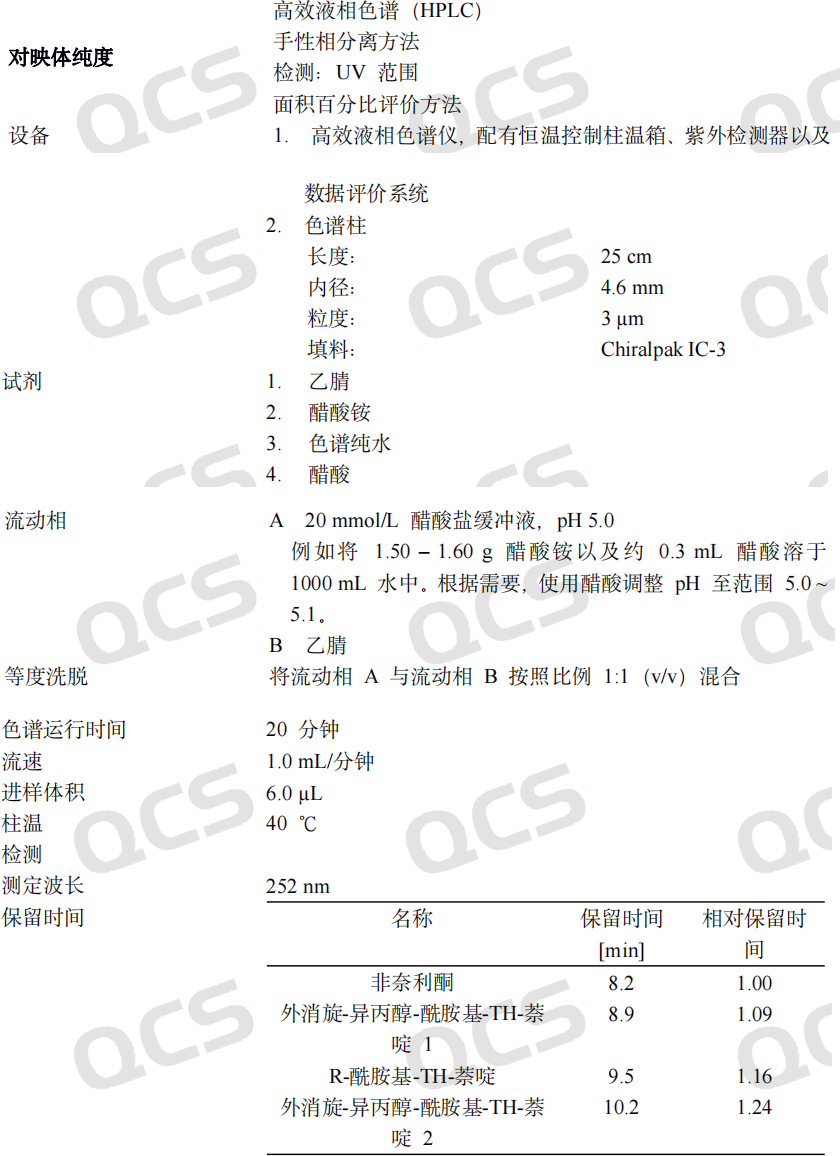
Figure 6: Chiral analysis method of Finarenone enantiomers
06 Featured Impurities in Non Nelitazone
Based on customer needs and market feedback, the QCS Standard Substance Research and Development Center conducted a special project and scientific research on non naridone related impurity products in May last year. Currently, a total of 122 non naridone impurities are included on the QCS official website. Through careful study of the drug review report for non noridone circulating online, and based on factors related to the synthesis process of raw materials and drug stability, the QCS R&D Center selected about 27 necessary impurities from a variety of possible impurity structures to help researchers complete impurity research work more quickly.
The preferred list of 27 essential impurities can be obtained by contacting our marketing and sales representatives if any customers require it. The existing information in our center is shown in Figure 7. Please feel free to contact us for more information.

Figure 7: Summary of relevant standards and impurity information for fenalidomide
Long press the recognition QR code to view a list of all impurities!

01 Introduction
Today, we will share the research on impurities related to non steroidal selective mineralocorticoid receptor antagonists -
Finarenone. Currently, there are 2 registration approval numbers for non phenanthrone preparations and raw materials in China, including 2 formulation approval numbers and no raw material approval number.

02 Introduction to Finarenone
Finelinone is a non steroidal selective mineralocorticoid receptor antagonist. In preclinical studies, it has been shown that Finelinone can block the harmful effects caused by excessive activation of the mineralocorticoid receptor, thus it can be used to treat chronic kidney disease related to type 2 diabetes, and reduce the risk of continuous decline in the estimated glomerular filtration rate (eGFR) and end-stage renal disease.
At present, the QCS official website has collected a total of 122 impurities of non noridone (scan the QR code at the end of the article to view the list of all impurities).Our laboratory has conducted liquid chromatography localization research on some popular impurities in the company's inventory using the HPLC chromatographic conditions used in the import registration standard of "Non noridone Tablets" (standard number JX20220072). The impurity code of the tablet and the structure information of the customer's key research impurities are shown in Figure 1.

Figure 1: List of impurities focused on research by nonelidone-related customers
03 Typical chromatograms and measured results of related impurities
Referring to the relevant import registration standards, two detection conditions are provided for the substance inspection items of non noridone tablets. The relevant information and typical chromatogram are shown in Figure 2. In this study, the QCS R&D center referred to the HPLC detection conditions and made appropriate adjustments to the relevant chromatographic conditions. The HPLC conditions and chromatogram results used in this study are shown in Figure 3.

Figure 2: Typical chromatogram of mixed injection of Finarenoneraw material and impurities

Figure 3: Typical results of QCS R&D center's measured mixed injection of non noridone raw materials and impurities
Although the stationary phase of the chromatography column in this study was not exactly the same as the standard, the three impurity codes BAY1814286, BAY1040818, and BAY1814288 included in the import registration standard for "Fenerlidone Tablets" (standard number JX20220072) were used as the judgment basis. In the actual liquid phase test, the elution order was consistent with the relevant standards, and the measured relative retention time was basically consistent with the data in the standard. It can be considered that the chromatographic results of the import registration standard were basically reproduced in this study.
Liquid phase results of 04 non-standard impurities
Using this liquid chromatography condition, the QCS R&D center conducted tests on multiple impurities that were not included in the standard but actually needed to be studied under the same conditions, in order to provide researchers with richer research results. In this study, the QCS R&D center collected a total of 10 impurity products in stock and conducted a series of studies using the same liquid chromatography conditions. The relative retention time and other data of all impurity products were calculated (as shown in Figure 4).

Figure 4: Localization spectra and data summary of 7 non registered
standard impurities under the same liquid chromatography conditions
The focus of the relevant import registration standard documents referred to this time is on the study of process impurities and degradation impurities, and no research has been conducted on chiral impurities. Therefore, the previous text lacks the ability to distinguish chiral impurities, especially those related to enantiomers.
From the data in Figure 4, it can be seen that under this chromatographic condition, when injecting API and multiple impurities simultaneously, three impurity products have the same retention time, namely RM-F260000, RM-F260004, and RM-F260011 (structural formula shown in Figure 5). The above three enantiomeric products cannot be distinguished under this condition.

Figure 5: Structural information of RM-F260000&04&11
05 Analysis Method for Chiral Impurities of Non Nelitazone
However, for drug molecules with chiral centers, the study of their chiral impurities is inevitably an unavoidable issue. The catalogue numbers provided by the QCS R&D center are RM-F260000, RM-F260004, and RM-F260011, corresponding to (S) - Non Naridone standard (API, optically pure), (R) - Non Naridone standard (API enantiomer, optically pure), and racemic Non Naridone standard (racemic), respectively. The above three products can help researchers use them as controls in chiral development and detection work.
This article will not elaborate on the development of chiral analysis methods. Instead, we will share relevant information on the conditions of the corresponding isomer analysis methods for non naridone with researchers for reference (see Figure 6, source: Production process information table for non naridone tablets (JXHS210017-8)).

Figure 6: Chiral analysis method of Finarenone enantiomers
06 Featured Impurities in Non Nelitazone
Based on customer needs and market feedback, the QCS Standard Substance Research and Development Center conducted a special project and scientific research on non naridone related impurity products in May last year. Currently, a total of 122 non naridone impurities are included on the QCS official website. Through careful study of the drug review report for non noridone circulating online, and based on factors related to the synthesis process of raw materials and drug stability, the QCS R&D Center selected about 27 necessary impurities from a variety of possible impurity structures to help researchers complete impurity research work more quickly.
The preferred list of 27 essential impurities can be obtained by contacting our marketing and sales representatives if any customers require it. The existing information in our center is shown in Figure 7. Please feel free to contact us for more information.

Figure 7: Summary of relevant standards and impurity information for fenalidomide
Long press the recognition QR code to view a list of all impurities!

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号