Time:2025-08-04

Piperacillin, a semi-synthetic penicillin antibiotic, exhibits broad-spectrum antibacterial activity by inhibiting bacterial cell wall synthesis. It demonstrates potent antimicrobial effects against Enterobacteriaceae bacteria including Escherichia coli, Proteus spp., Serratia spp., Klebsiella spp., Enterobacter spp., Citrobacter spp., Salmonella spp., and Shigella spp., as well as other Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter spp., Haemophilus influenzae, and Neisseria spp. The drug also shows activity against Enterococcus spp., Group A/B hemolytic streptococci, Streptococcus pneumoniae, and non-penicillinase-producing Staphylococcus. Additionally, many anaerobic bacteria like Bacteroides fragilis and Clostridium spp. exhibit susceptibility to pipracillin.
Piperacillin sodium is administered via intramuscular and intravenous routes, with widespread distribution in body fluids and tissues. Like other newer penicillins, it is excreted through renal and biliary mechanisms. The primary elimination pathway involves glomerular filtration, resulting in elevated concentrations of unchanged compounds in urine. Piperacillin has been approved for treating severe infections caused by specific sensitive bacterial strains, including those occurring in the abdominal cavity, urinary tract, gynecological system, lower respiratory tract, skin and dermal structures, bones and joints, as well as gonococcal infections and sepsis.
Overall, piperacillin has a higher degree of activity than other penicillins. Evidence from prospective studies suggests that piperacillin is an effective drug for treating patients with infections caused by susceptible microbes.
I. R&D Background
During production, piperacillin sodium raw materials are susceptible to degradation caused by pH levels, temperature fluctuations, and light exposure, leading to the formation of various impurities. Notably, the side chain of piperacillin sodium contains three amide bonds that are prone to hydrolysis and cleavage. The European Pharmacopoeia (EP) provides comprehensive research on impurities in piperacillin sodium. As one of our company's best-selling impurity series, we conducted thorough studies on 14 specific impurities using EP methods to enhance quality control. The structural details are illustrated in Figure 1.
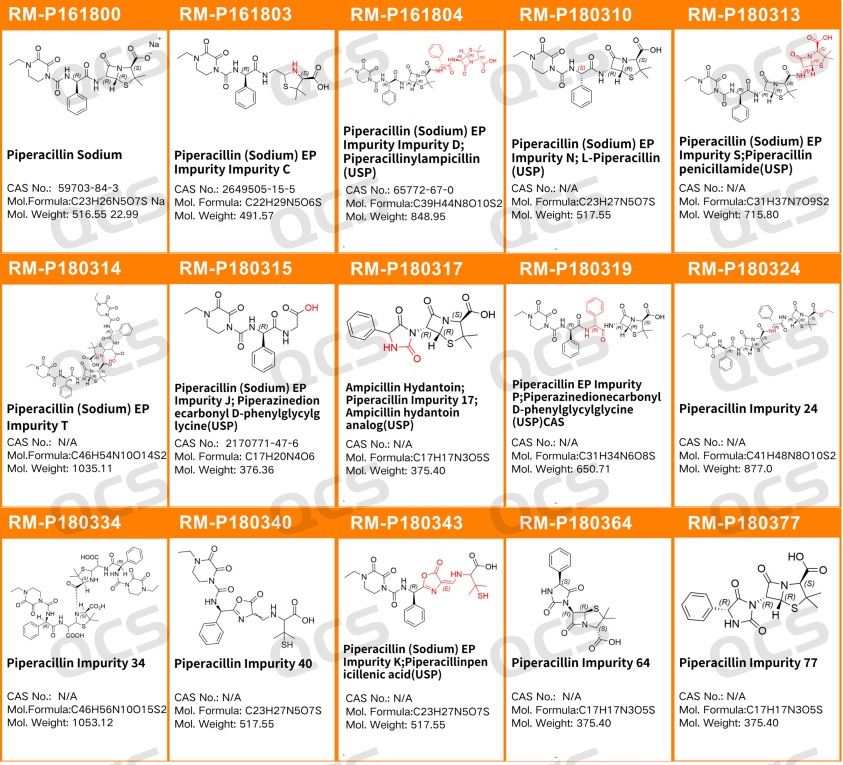
Figure 1: Structure of piperacillin sodium and piperacillin sodium related substances
II.HPLC method study
After the qualitative determination of piperacillin sodium impurities by NMR and mass spectrometry, our company carried out HPLC liquid phase analysis of impurities according to the EP piperacillin sodium related substances method, and the results detected according to the established scheme are shown in Figure 2 and the following table.
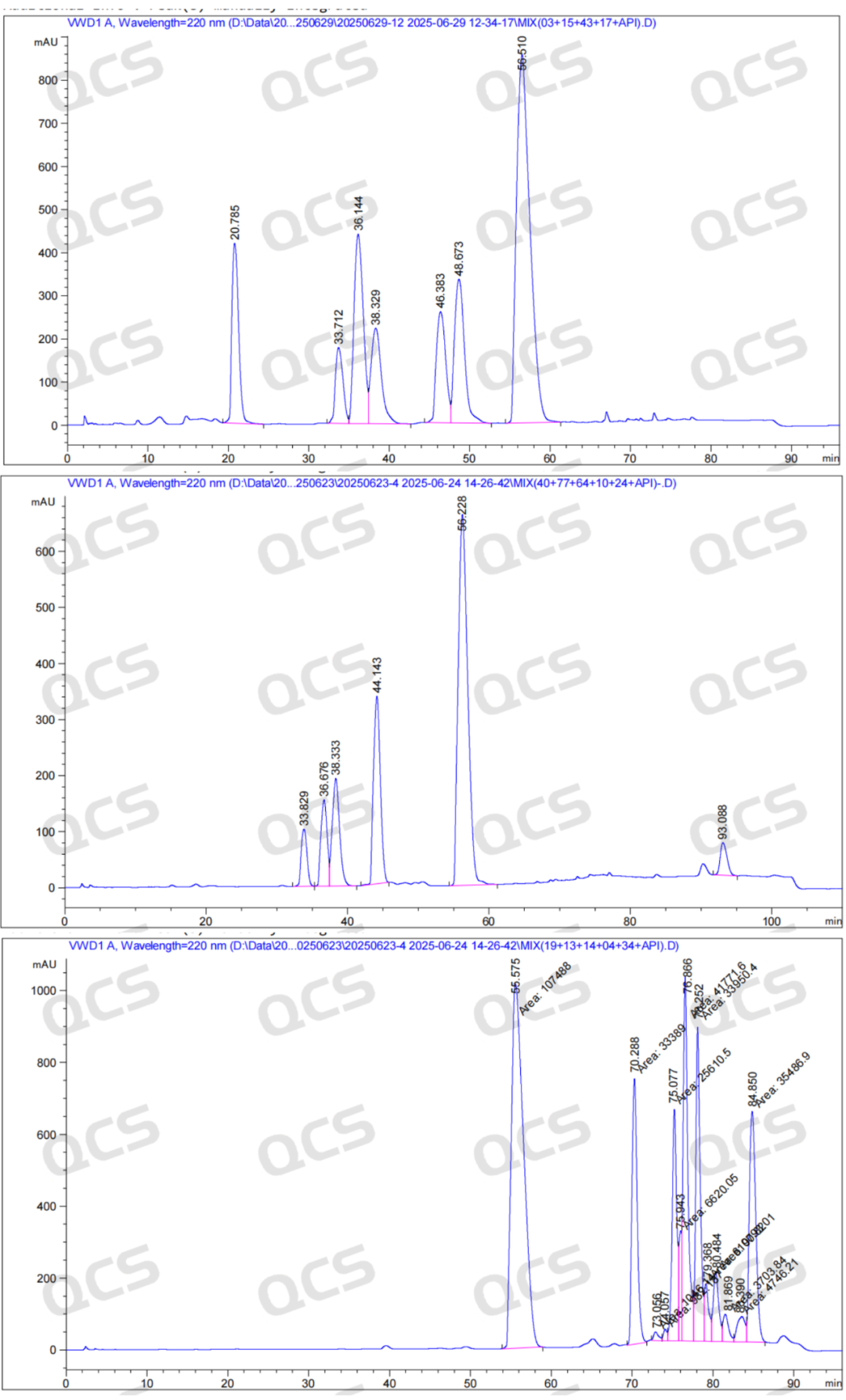
Figure 2: Sametrol impurity location diagram
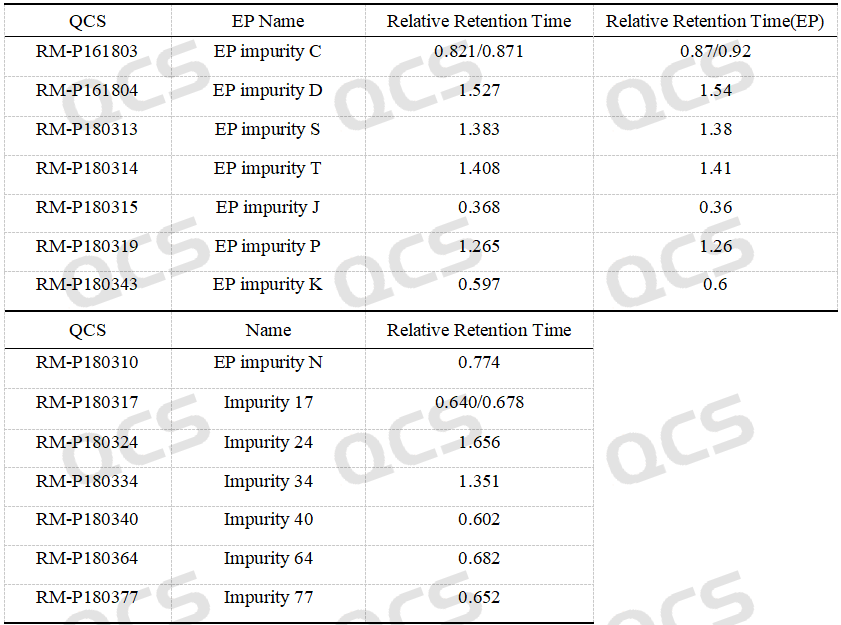
Table 1: Relative retention time of impurities of piperacillin sodium
According to the above results, we can see that the relative retention time of each impurity is within ±0.05 of the standard requirements, and our company has successfully realized the central control and monitoring of 14 kinds of piperacillin sodium related substances.
thematic knowledge

Figure 3: Schematic diagram of resonance anti-bond orbital weakening of β-lactam
β The amide bond in the lactam is highly reactive, which is the key factor for the biological activity of this structure, but also brings great challenges in the research process.
According to traditional chemical principles, amide bonds exhibit a resonance effect between their C=O and C–N bonds (π resonance). This means electrons between these two atoms "jump" between multiple structural configurations (Figures 5, 6, 7), forming a more stable electron cloud structure that reduces the reactivity of conventional amide bonds. This explains why such bonds are relatively inert – you can safely handle amide-containing samples in laboratory settings without worrying about decomposition.
However, beyond this π resonance, another scenario occurs where the lone electrons of oxygen atoms (electrons not involved in bonding) interfere with the C–N bond, weakening it (as shown in Figure 8 through the interaction between oxygen's non-bonding orbitals and the σ* orbital of the C–N bond). This disrupts the resonance equilibrium between 5-6-7, causing the molecule to remain more predominantly in higher-energy structures like structure 5.
From the perspective of molecular orbitals, when a molecular structure becomes "distorted" —such as nitrogen atoms no longer being flat or C-N bonds rotating—the previously mentioned resonance effect is significantly weakened. With reduced resonance, the molecule tends to adopt a more "traditional" structure like Figure 5. This elongates the C-N bond while theoretically shortening the C=O bond, thereby enhancing reactivity.
This is also the reason why β-lactam structure is easy to react with water, alcohols, amines and other nucleophilic reagents, which are commonly used reagents in chromatography, resulting in the degradation of this kind of β-lactam samples in liquid phase related experiments.
III. Summary
Piperacillin sodium requires stringent impurity control, particularly given its β-lactam ring structure—which is highly sensitive to heat, acids, bases, and organic solvents—making it prone to ring-opening during synthesis or storage. The resulting open-ring products may not only bind to proteins to form antigens that trigger allergic reactions, but could also undergo further polymerization, thereby increasing immunological risks.
Our company employs standardized methods to rigorously control the quality of piperacillin sodium impurity reference materials. We have developed customized quality control protocols tailored to each impurity's structural characteristics and stability profiles. Although certain impurities' purity levels may be challenging to compare with others due to structural stability constraints, we maintain strict controls throughout production, testing, and storage processes to ensure consistent quality and full traceability.
Piperacillin sodium impurity is one of our best-selling products, with complete chromatography, quality control data, and support for customized development. Welcome to consult more related products and services.



Piperacillin, a semi-synthetic penicillin antibiotic, exhibits broad-spectrum antibacterial activity by inhibiting bacterial cell wall synthesis. It demonstrates potent antimicrobial effects against Enterobacteriaceae bacteria including Escherichia coli, Proteus spp., Serratia spp., Klebsiella spp., Enterobacter spp., Citrobacter spp., Salmonella spp., and Shigella spp., as well as other Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter spp., Haemophilus influenzae, and Neisseria spp. The drug also shows activity against Enterococcus spp., Group A/B hemolytic streptococci, Streptococcus pneumoniae, and non-penicillinase-producing Staphylococcus. Additionally, many anaerobic bacteria like Bacteroides fragilis and Clostridium spp. exhibit susceptibility to pipracillin.
Piperacillin sodium is administered via intramuscular and intravenous routes, with widespread distribution in body fluids and tissues. Like other newer penicillins, it is excreted through renal and biliary mechanisms. The primary elimination pathway involves glomerular filtration, resulting in elevated concentrations of unchanged compounds in urine. Piperacillin has been approved for treating severe infections caused by specific sensitive bacterial strains, including those occurring in the abdominal cavity, urinary tract, gynecological system, lower respiratory tract, skin and dermal structures, bones and joints, as well as gonococcal infections and sepsis.
Overall, piperacillin has a higher degree of activity than other penicillins. Evidence from prospective studies suggests that piperacillin is an effective drug for treating patients with infections caused by susceptible microbes.
I. R&D Background
During production, piperacillin sodium raw materials are susceptible to degradation caused by pH levels, temperature fluctuations, and light exposure, leading to the formation of various impurities. Notably, the side chain of piperacillin sodium contains three amide bonds that are prone to hydrolysis and cleavage. The European Pharmacopoeia (EP) provides comprehensive research on impurities in piperacillin sodium. As one of our company's best-selling impurity series, we conducted thorough studies on 14 specific impurities using EP methods to enhance quality control. The structural details are illustrated in Figure 1.

Figure 1: Structure of piperacillin sodium and piperacillin sodium related substances
II.HPLC method study
After the qualitative determination of piperacillin sodium impurities by NMR and mass spectrometry, our company carried out HPLC liquid phase analysis of impurities according to the EP piperacillin sodium related substances method, and the results detected according to the established scheme are shown in Figure 2 and the following table.

Figure 2: Sametrol impurity location diagram

Table 1: Relative retention time of impurities of piperacillin sodium
According to the above results, we can see that the relative retention time of each impurity is within ±0.05 of the standard requirements, and our company has successfully realized the central control and monitoring of 14 kinds of piperacillin sodium related substances.
thematic knowledge

Figure 3: Schematic diagram of resonance anti-bond orbital weakening of β-lactam
β The amide bond in the lactam is highly reactive, which is the key factor for the biological activity of this structure, but also brings great challenges in the research process.
According to traditional chemical principles, amide bonds exhibit a resonance effect between their C=O and C–N bonds (π resonance). This means electrons between these two atoms "jump" between multiple structural configurations (Figures 5, 6, 7), forming a more stable electron cloud structure that reduces the reactivity of conventional amide bonds. This explains why such bonds are relatively inert – you can safely handle amide-containing samples in laboratory settings without worrying about decomposition.
However, beyond this π resonance, another scenario occurs where the lone electrons of oxygen atoms (electrons not involved in bonding) interfere with the C–N bond, weakening it (as shown in Figure 8 through the interaction between oxygen's non-bonding orbitals and the σ* orbital of the C–N bond). This disrupts the resonance equilibrium between 5-6-7, causing the molecule to remain more predominantly in higher-energy structures like structure 5.
From the perspective of molecular orbitals, when a molecular structure becomes "distorted" —such as nitrogen atoms no longer being flat or C-N bonds rotating—the previously mentioned resonance effect is significantly weakened. With reduced resonance, the molecule tends to adopt a more "traditional" structure like Figure 5. This elongates the C-N bond while theoretically shortening the C=O bond, thereby enhancing reactivity.
This is also the reason why β-lactam structure is easy to react with water, alcohols, amines and other nucleophilic reagents, which are commonly used reagents in chromatography, resulting in the degradation of this kind of β-lactam samples in liquid phase related experiments.
III. Summary
Piperacillin sodium requires stringent impurity control, particularly given its β-lactam ring structure—which is highly sensitive to heat, acids, bases, and organic solvents—making it prone to ring-opening during synthesis or storage. The resulting open-ring products may not only bind to proteins to form antigens that trigger allergic reactions, but could also undergo further polymerization, thereby increasing immunological risks.
Our company employs standardized methods to rigorously control the quality of piperacillin sodium impurity reference materials. We have developed customized quality control protocols tailored to each impurity's structural characteristics and stability profiles. Although certain impurities' purity levels may be challenging to compare with others due to structural stability constraints, we maintain strict controls throughout production, testing, and storage processes to ensure consistent quality and full traceability.
Piperacillin sodium impurity is one of our best-selling products, with complete chromatography, quality control data, and support for customized development. Welcome to consult more related products and services.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号