Time:2025-05-15

Mupirocin (Mupirocin), also known as pseudomonas acid A (Pseudomonas acid A) and trans-pseudomonas acid, is a broad-spectrum antibiotic produced by the fermentation of fluorescent pseudomonas. It has a special structure and is not related to other antibiotics.
The primary mechanism of mupirocin is to bind to the RNA and proteins of Staphylococcus aureus and Escherichia coli, thereby blocking the supply of isoleucine. This leads to the cessation of all isoleucine-containing protein synthesis within the bacteria, resulting in their death. Additionally, mupirocin can mildly inhibit the synthesis of DNA and cell wall peptidoglycan, making it effective against common skin bacterial infections.
I. R&D Background
The raw material of mupirocin is susceptible to degradation during production due to factors such as pH, temperature, and light exposure, leading to the formation of various impurities. The USP standards for mupirocin ointment have conducted detailed research on these impurities. Our center focuses on overcoming the challenges posed by mupirocin impurities. To better control the quality of these impurities, we have conducted qualitative studies on the 7 standard impurities of mupirocin using the USP method for mupirocin ointment, ensuring customer confidence in its use. The following figure (Figure 1) illustrates the correspondence between the impurities listed in the USP standards for mupirocin ointment and those in the EP standards, along with the corresponding RRT and QCS item numbers.
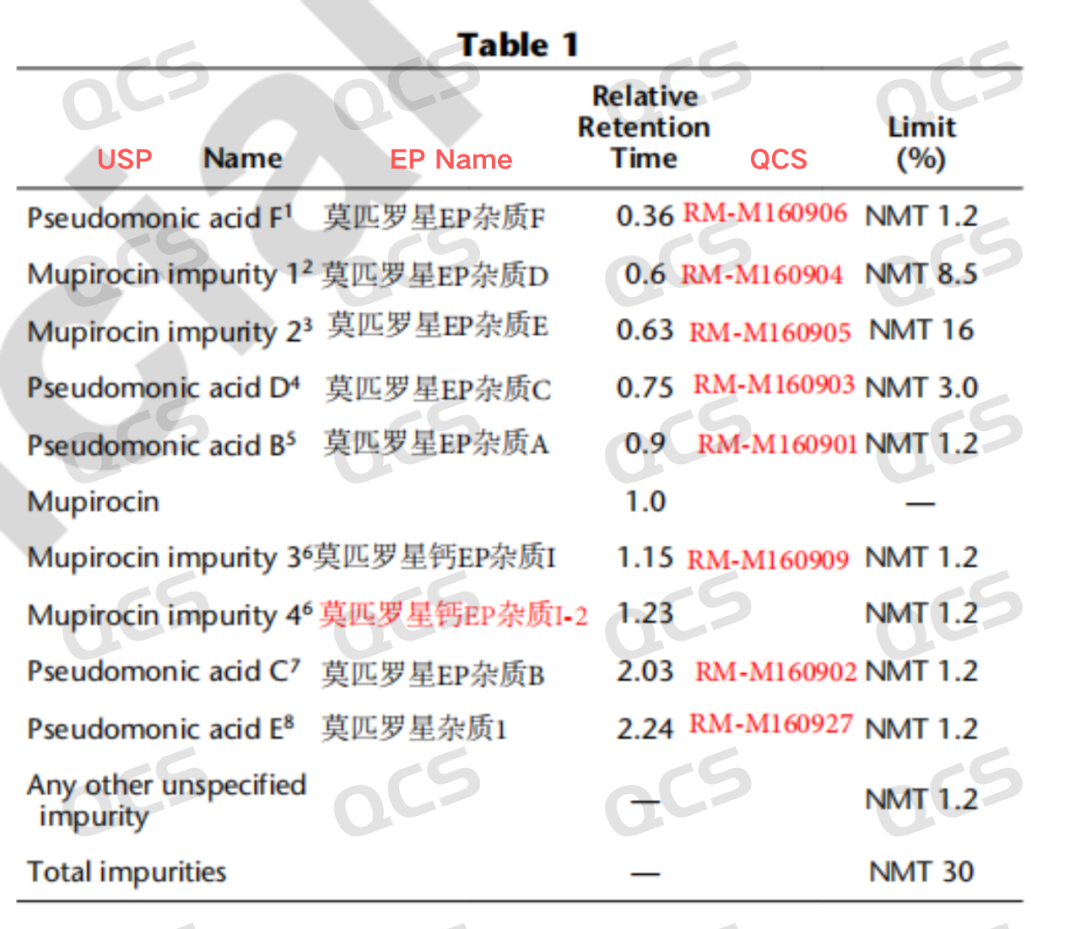
Figure 1: Correspondence between impurity names and lot numbers in USP/EP standards
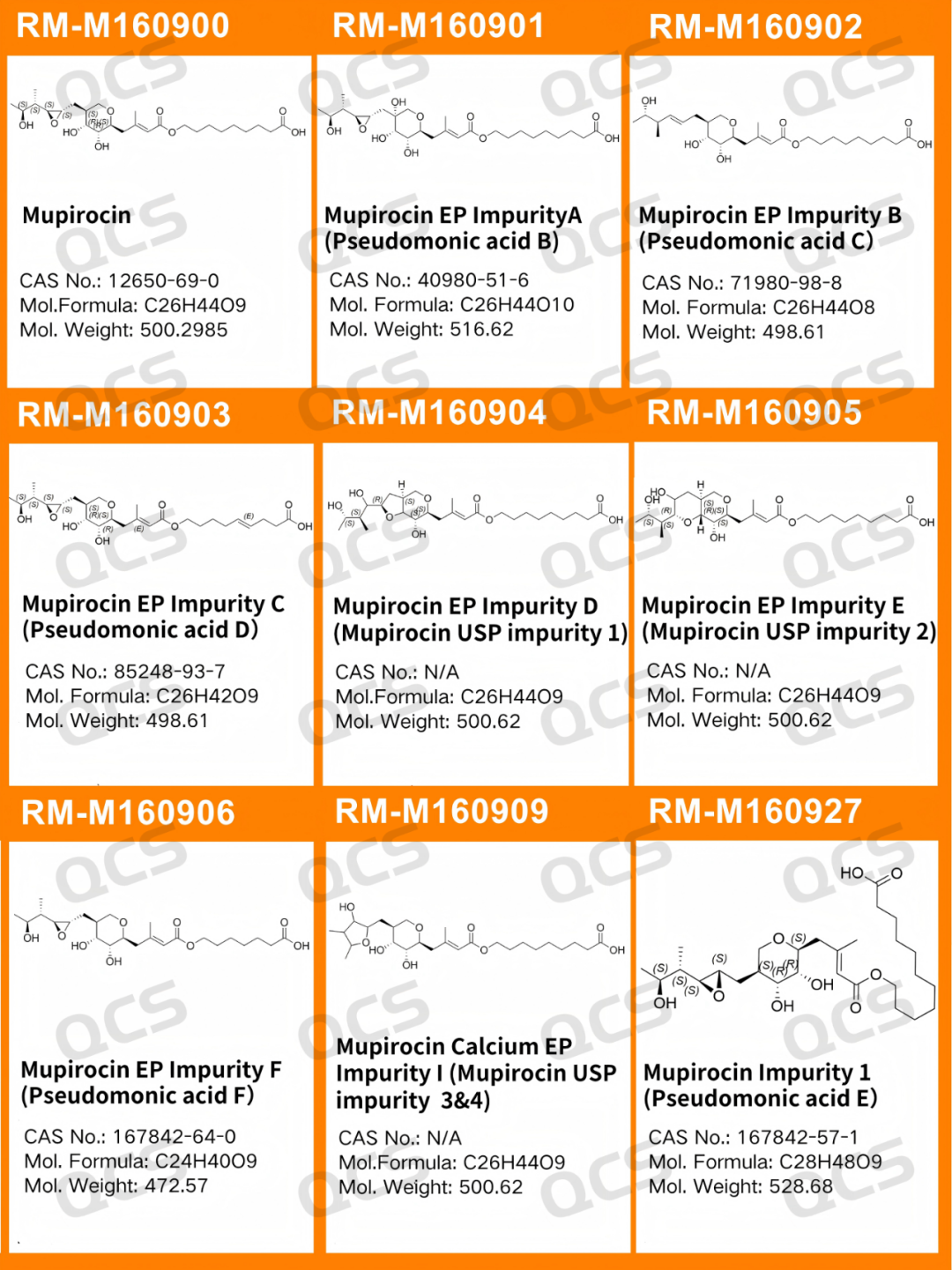
Figure 2: Structural information of the standard impurities included in mupirocin
2, preliminary research and conclusions
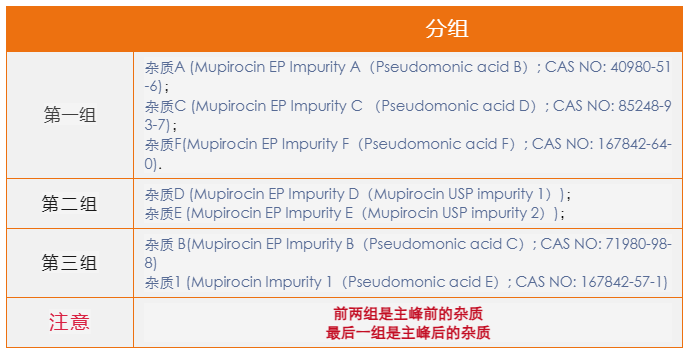
According to the cream standard of USP, we found that the RRT of impurity DE was very close. In order to better present the separation and localization of impurity, we divided the impurity into three groups for separate mixing study.
The results of the test according to the established scheme are shown in Figure 3-5.
Group 1: The results of qualitative localization study of mupirocin and impurities A, C and F (Figure 3)
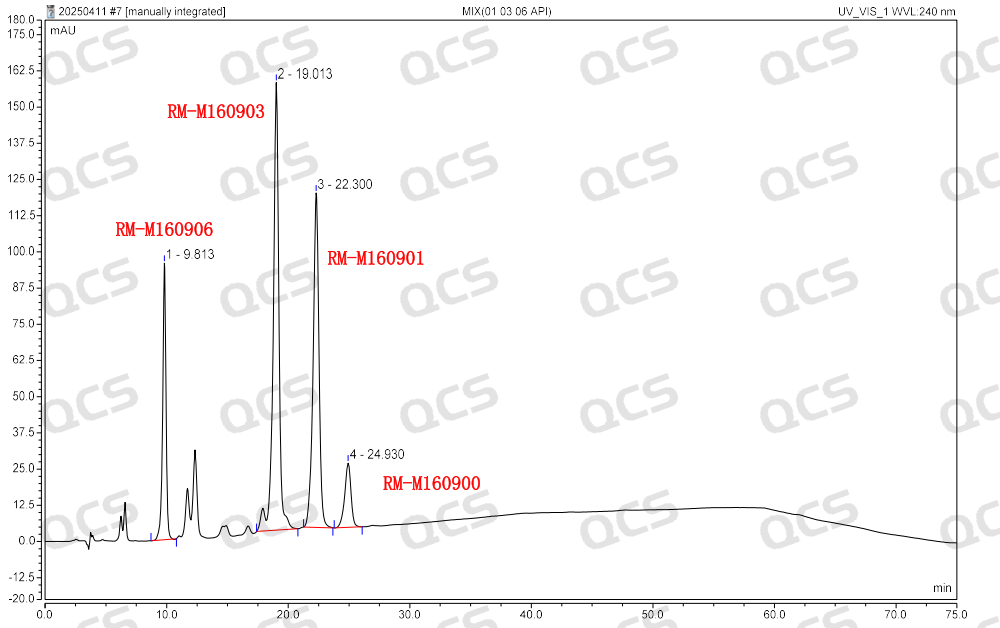
Figure 3: Location of mupirocin and impurities A, C and F
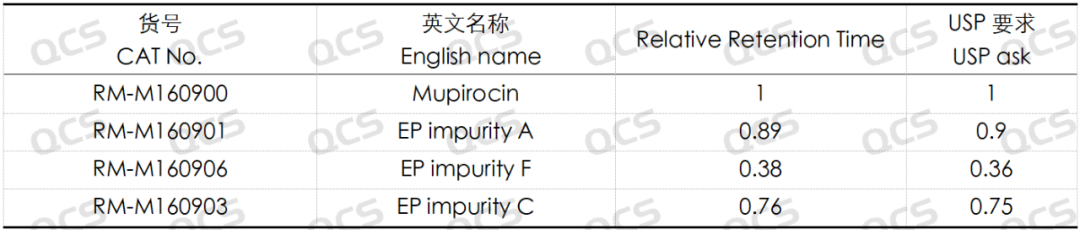
Table 1: Summary of the relative retention times of mupirocin and impurities A, C and F
Group 2: The results of qualitative localization of mupirocin and impurities D and E (Figure 4)
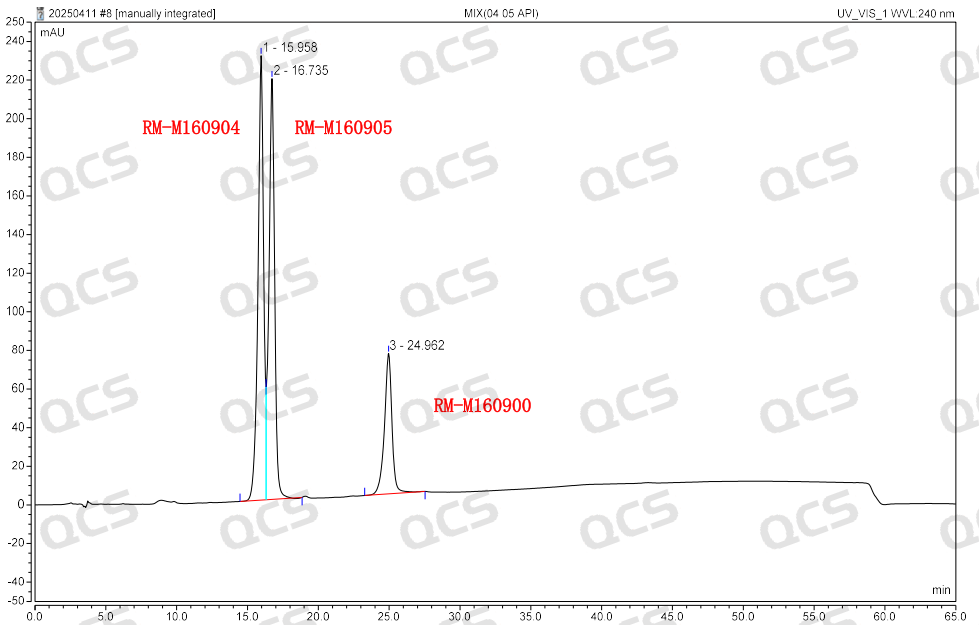
Figure 4: Location of mupirocin and impurities D and E
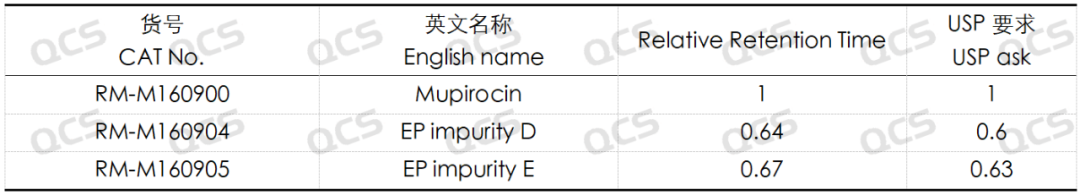
Table 2: Relative retention times of mupirocin and mupirocin EP impurities D and impurity E
Group 3: The results of qualitative localization of mupirocin and impurities B and impurity 1 (Figure 5)
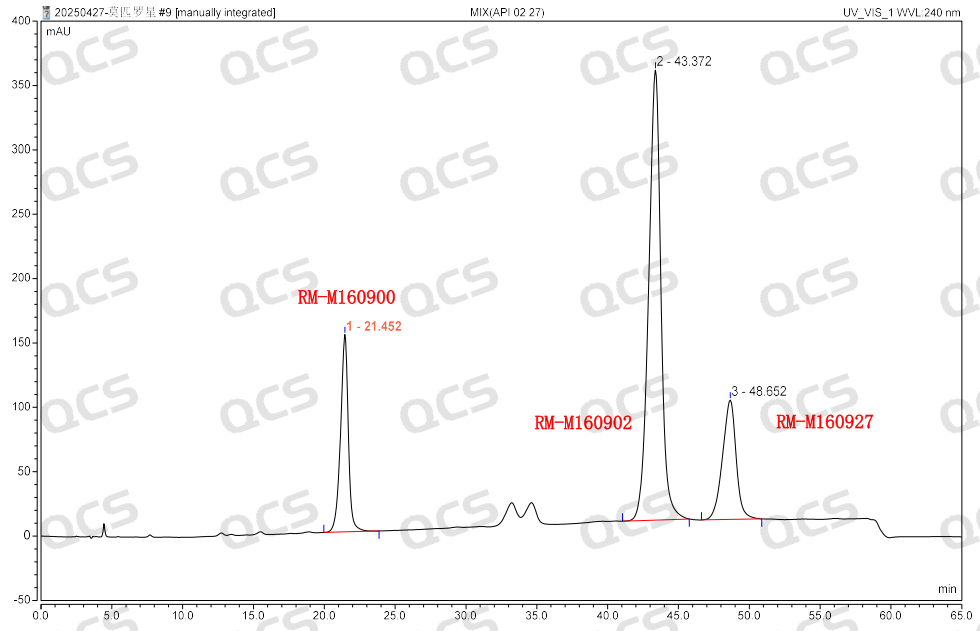
Figure 5: Location of mupirocin and impurities B and impurity 1
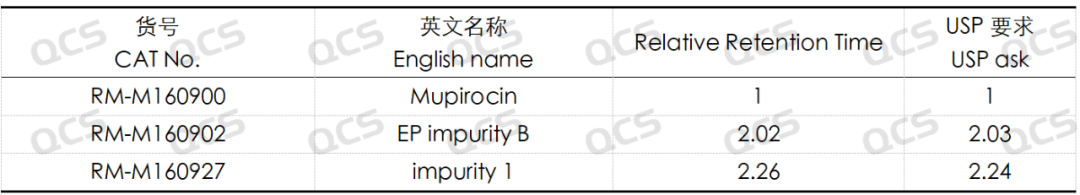
Table 3: Relative retention times of mupirocin and impurities B and impurity 1
According to the above results, we can see that the relative retention time of each impurity is within ±0.05 of the standard requirements, and our company has successfully realized the central control and monitoring of 7 mupirocin related substances.
3.summary
Through the above verification data, our center has finally completed the development of impurities of this variety and the corresponding impurity verification work. We welcome customers to consult and purchase. If customers are interested in our verification data, they can ask us for relevant data.



Mupirocin (Mupirocin), also known as pseudomonas acid A (Pseudomonas acid A) and trans-pseudomonas acid, is a broad-spectrum antibiotic produced by the fermentation of fluorescent pseudomonas. It has a special structure and is not related to other antibiotics.
The primary mechanism of mupirocin is to bind to the RNA and proteins of Staphylococcus aureus and Escherichia coli, thereby blocking the supply of isoleucine. This leads to the cessation of all isoleucine-containing protein synthesis within the bacteria, resulting in their death. Additionally, mupirocin can mildly inhibit the synthesis of DNA and cell wall peptidoglycan, making it effective against common skin bacterial infections.
I. R&D Background
The raw material of mupirocin is susceptible to degradation during production due to factors such as pH, temperature, and light exposure, leading to the formation of various impurities. The USP standards for mupirocin ointment have conducted detailed research on these impurities. Our center focuses on overcoming the challenges posed by mupirocin impurities. To better control the quality of these impurities, we have conducted qualitative studies on the 7 standard impurities of mupirocin using the USP method for mupirocin ointment, ensuring customer confidence in its use. The following figure (Figure 1) illustrates the correspondence between the impurities listed in the USP standards for mupirocin ointment and those in the EP standards, along with the corresponding RRT and QCS item numbers.

Figure 1: Correspondence between impurity names and lot numbers in USP/EP standards

Figure 2: Structural information of the standard impurities included in mupirocin
2, preliminary research and conclusions
According to the cream standard of USP, we found that the RRT of impurity DE was very close. In order to better present the separation and localization of impurity, we divided the impurity into three groups for separate mixing study.

The results of the test according to the established scheme are shown in Figure 3-5.
Group 1: The results of qualitative localization study of mupirocin and impurities A, C and F (Figure 3)

Figure 3: Location of mupirocin and impurities A, C and F

Table 1: Summary of the relative retention times of mupirocin and impurities A, C and F
Group 2: The results of qualitative localization of mupirocin and impurities D and E (Figure 4)

Figure 4: Location of mupirocin and impurities D and E

Table 2: Relative retention times of mupirocin and mupirocin EP impurities D and impurity E
Group 3: The results of qualitative localization of mupirocin and impurities B and impurity 1 (Figure 5)

Figure 5: Location of mupirocin and impurities B and impurity 1

Table 3: Relative retention times of mupirocin and impurities B and impurity 1
According to the above results, we can see that the relative retention time of each impurity is within ±0.05 of the standard requirements, and our company has successfully realized the central control and monitoring of 7 mupirocin related substances.
3.summary
Through the above verification data, our center has finally completed the development of impurities of this variety and the corresponding impurity verification work. We welcome customers to consult and purchase. If customers are interested in our verification data, they can ask us for relevant data.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号