Time:2025-06-25

Salmeterol (Salmeterol) is a long-acting adrenergic receptor agonist, mainly used in the treatment of asthma and chronic bronchitis. Salmeterol was first developed by Glaxo Company in the United States, and was made into an aerosol with 5-hydroxy naphthyl carboxylic acid and salmeterol.
Our company has conducted specialized research and development on various official impurities of salmeterol, including EP impurities D, E, F, G, and others. We have successfully developed a series of impurity reference substances for salmeterol. These products are characterized by high purity, comprehensive spectra, and compliance with pharmacopoeia standards, making them widely used in customers' quality research, method development, and registration processes. Additionally, we offer customized services to meet the diverse needs at different stages of R&D.
I. R&D Background
The active pharmaceutical ingredient (API) salmeterol is susceptible to degradation and the formation of various impurities during production and storage due to external factors such as pH, temperature, and light exposure. Currently, both the European Pharmacopoeia (EP) and the United States Pharmacopeia (USP) have included multiple impurities in the quality standards for salmeterol, clearly defining their limits and testing methods, highlighting the importance of controlling these impurities.
As one of the key products in our company's popular impurity series, the study of salmeterol-related impurities is of significant importance for quality control. To enhance the identification and monitoring of salmeterol impurities, we have conducted a systematic study on four impurities —— namely, impurities D, E, F, and G listed in the EP, strictly following the methods outlined in the USP pharmacopoeia. This ensures that these impurities are effectively monitored during the production and quality control of salmeterol raw materials.
There is a certain correspondence between the impurities of Sametrol included in EP and USP, as shown in Figure 1.
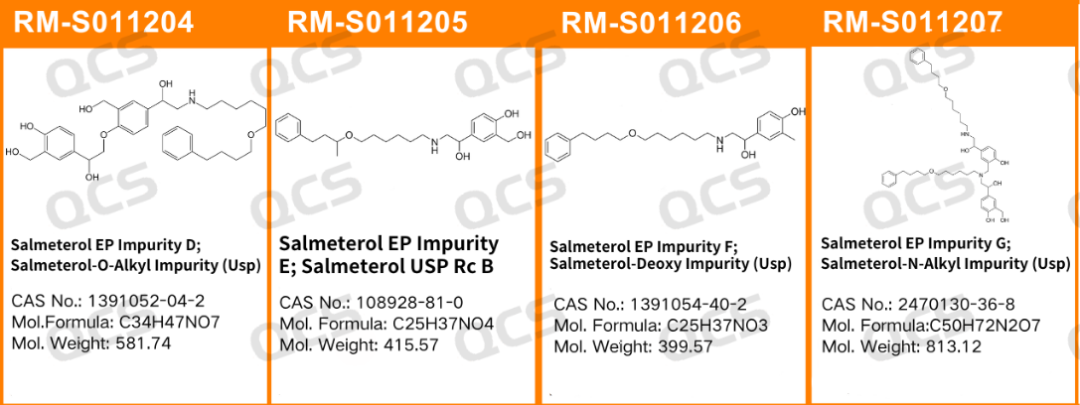
Figure 1: Correspondence between Chametrol EP and USP impurities
2. Study of HPLC method
Following the qualitative confirmation of the impurities in Sametrol via NMR and MS, our company conducted HPLC analysis on the impurity samples according to the standard methods outlined in the USP Pharmacopoeia (see Figure 2). The chromatographic results obtained from the established analytical protocol are presented in Figure 3, and the summarized RRT data are shown in Table 1.
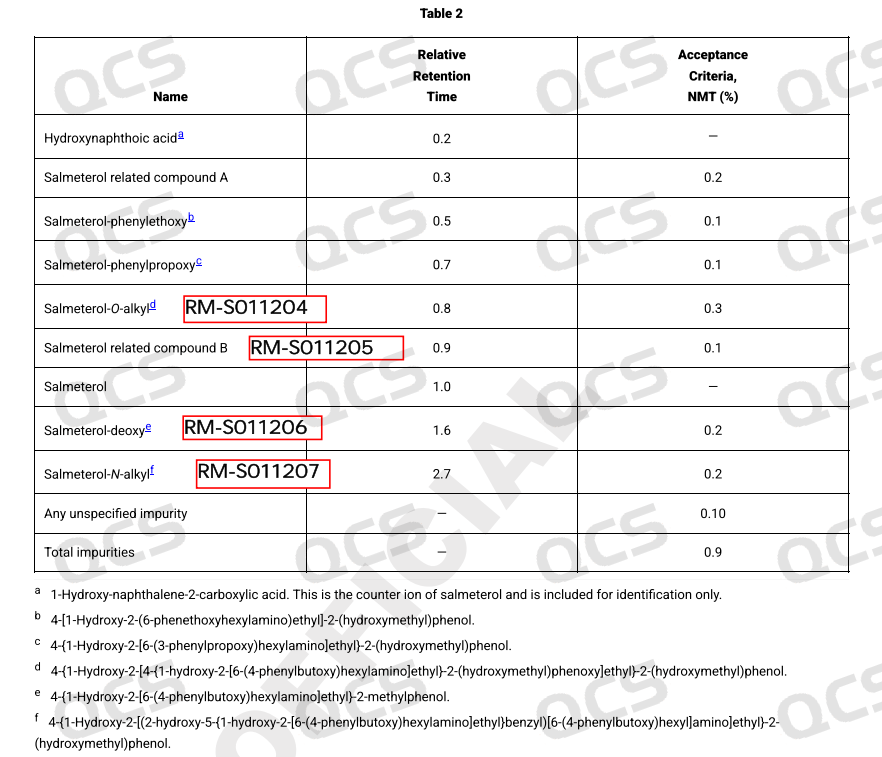
Figure 2: Sametrol USP related substance method
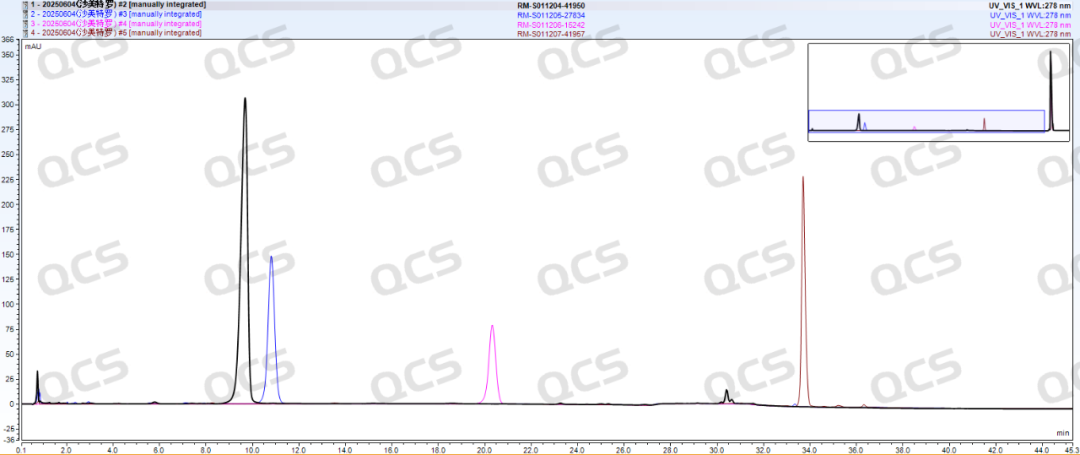
Figure 3: Localization of Salmeterol impurities under USP method
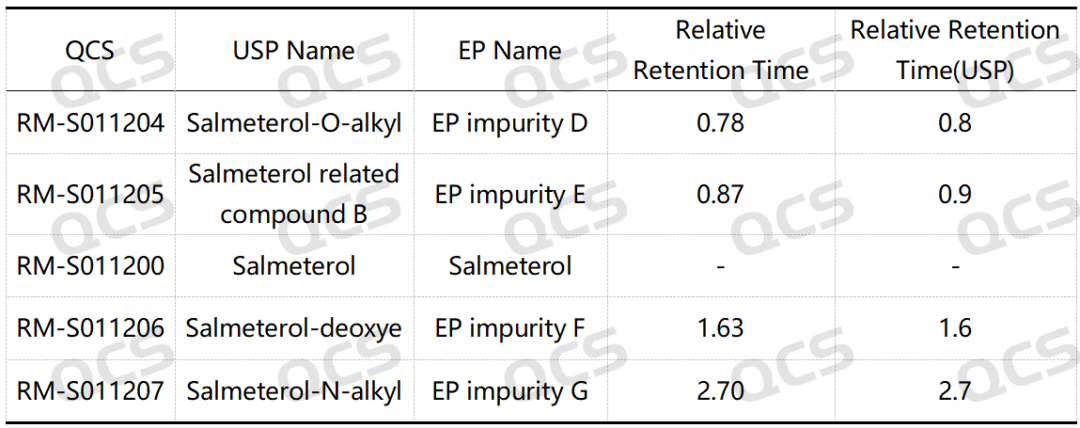
Table 1: Comparison of relative retention time (RRT) standards and measured impurities of Sametrol
According to the above results, we can see that the relative retention time (RRT) of each impurity is within ±0.05 of the standard requirements, and our company has successfully realized the central control and monitoring of 4 substances related to salmeterol.
Third, summary
Samentro impurities is one of the key products developed by our company. The existing products have perfect structural confirmation data and spectrum certificate, high purity, stable quality, competitive price, and can also provide customized services according to customer needs. Welcome to consult your corresponding business personnel for more relevant impurity information.



Salmeterol (Salmeterol) is a long-acting adrenergic receptor agonist, mainly used in the treatment of asthma and chronic bronchitis. Salmeterol was first developed by Glaxo Company in the United States, and was made into an aerosol with 5-hydroxy naphthyl carboxylic acid and salmeterol.
Our company has conducted specialized research and development on various official impurities of salmeterol, including EP impurities D, E, F, G, and others. We have successfully developed a series of impurity reference substances for salmeterol. These products are characterized by high purity, comprehensive spectra, and compliance with pharmacopoeia standards, making them widely used in customers' quality research, method development, and registration processes. Additionally, we offer customized services to meet the diverse needs at different stages of R&D.
I. R&D Background
The active pharmaceutical ingredient (API) salmeterol is susceptible to degradation and the formation of various impurities during production and storage due to external factors such as pH, temperature, and light exposure. Currently, both the European Pharmacopoeia (EP) and the United States Pharmacopeia (USP) have included multiple impurities in the quality standards for salmeterol, clearly defining their limits and testing methods, highlighting the importance of controlling these impurities.
As one of the key products in our company's popular impurity series, the study of salmeterol-related impurities is of significant importance for quality control. To enhance the identification and monitoring of salmeterol impurities, we have conducted a systematic study on four impurities —— namely, impurities D, E, F, and G listed in the EP, strictly following the methods outlined in the USP pharmacopoeia. This ensures that these impurities are effectively monitored during the production and quality control of salmeterol raw materials.
There is a certain correspondence between the impurities of Sametrol included in EP and USP, as shown in Figure 1.

Figure 1: Correspondence between Chametrol EP and USP impurities
2. Study of HPLC method
Following the qualitative confirmation of the impurities in Sametrol via NMR and MS, our company conducted HPLC analysis on the impurity samples according to the standard methods outlined in the USP Pharmacopoeia (see Figure 2). The chromatographic results obtained from the established analytical protocol are presented in Figure 3, and the summarized RRT data are shown in Table 1.

Figure 2: Sametrol USP related substance method

Figure 3: Localization of Salmeterol impurities under USP method

Table 1: Comparison of relative retention time (RRT) standards and measured impurities of Sametrol
According to the above results, we can see that the relative retention time (RRT) of each impurity is within ±0.05 of the standard requirements, and our company has successfully realized the central control and monitoring of 4 substances related to salmeterol.
Third, summary
Samentro impurities is one of the key products developed by our company. The existing products have perfect structural confirmation data and spectrum certificate, high purity, stable quality, competitive price, and can also provide customized services according to customer needs. Welcome to consult your corresponding business personnel for more relevant impurity information.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号