Time:2025-04-14


Today, we share the study on the stability of specific impurities in short-acting β2-adrenergic receptor agonist—salbutamol. Salbutamol (salbutamol) is a short-acting β2-adrenergic receptor agonist used as an asthma reliever, effectively inhibiting the release of allergens such as histamine and preventing bronchial spasms. Adding trace amounts of salbutamol to livestock feed can increase lean meat yield and conversion rate, while reducing fat content. However, its toxicity is much higher than that of ractopamine, which has the same function. It is suitable for treating bronchial asthma, wheezing bronchitis, bronchial spasms, emphysema, and other conditions.
Experimental scheme
In this experiment, our center carried out a study on the solution stability of three specific impurities of salbutamol according to the chromatographic conditions used in item "Related substances" of "SALBUTAMOL" variety in European Pharmacopoeia 11.0, and the sample number and structural formula used are shown in Figure 1 and Figure 2:
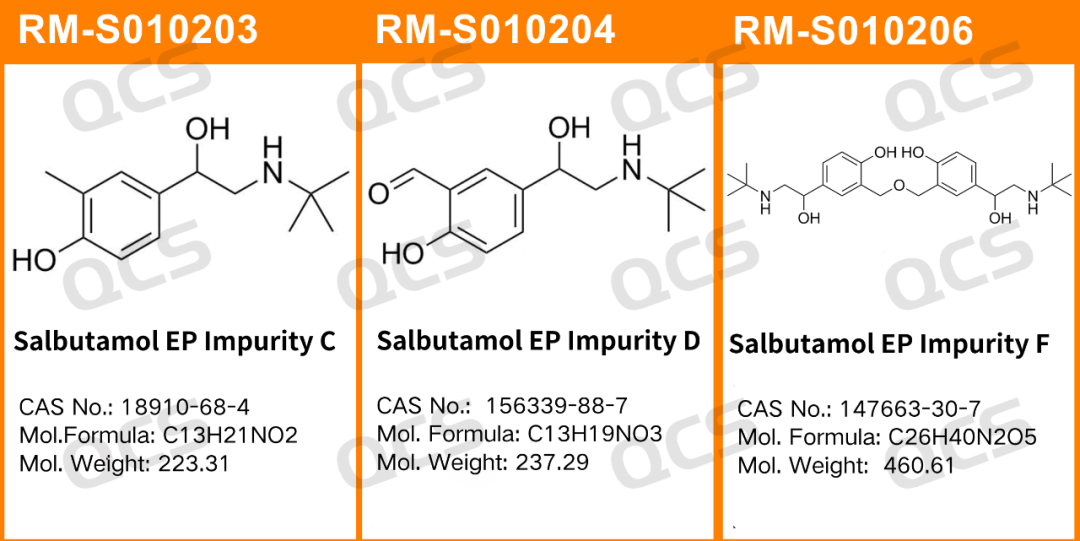
Figure 1: The impurity number and structure of the impurity used in this study
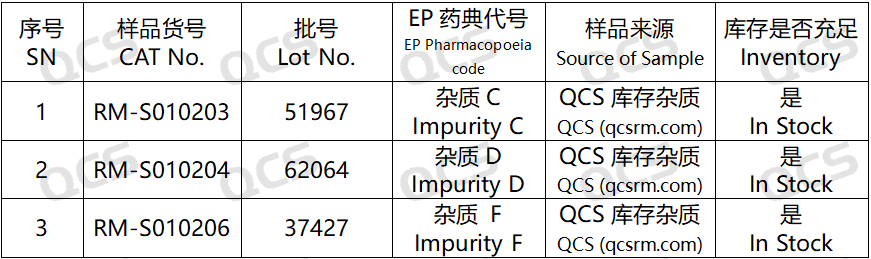
Figure 2: Standard inclusion of impurity codes and the correspondence between impurity numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-S010203 (EP impurity C; Salbutamol EP Impurity C; CAS No:18910-68-4), RM-S010204 (EP impurity D; Salbutamol EP Impurity D; CAS No:156339-88-7), and RM-S010206 (EP impurity F; Salbutamol EP Impurity F; CAS No:147663-30-7). These samples were placed in acidic, neutral, and alkaline solutions, respectively. They were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours. Subsequently, the samples were analyzed using the chromatographic conditions specified under "Related substances" in the "SALBUTAMOL" section of the 11.0 edition of the European Pharmacopoeia. The changes in the main peak area of the chromatogram over time were observed to determine the stability of the sample solutions.
Experiment conclusion
After testing, it was found that the main peak area of samples RM-S010203 (EP impurity C), RM-S010204 (EP impurity D), and RM-S010206 (EP impurity F) did not change significantly over 24 hours in acidic, neutral, and alkaline solutions, with relative standard deviations all less than 2.0%. Therefore, these three samples can be considered relatively stable during the 24-hour period in acidic, neutral, and alkaline solutions. The main peak area data for each detection point under different pH conditions for samples RM-S010203 (EP impurity C), RM-S010204 (EP impurity D), and RM-S010206 (EP impurity F) are as follows:
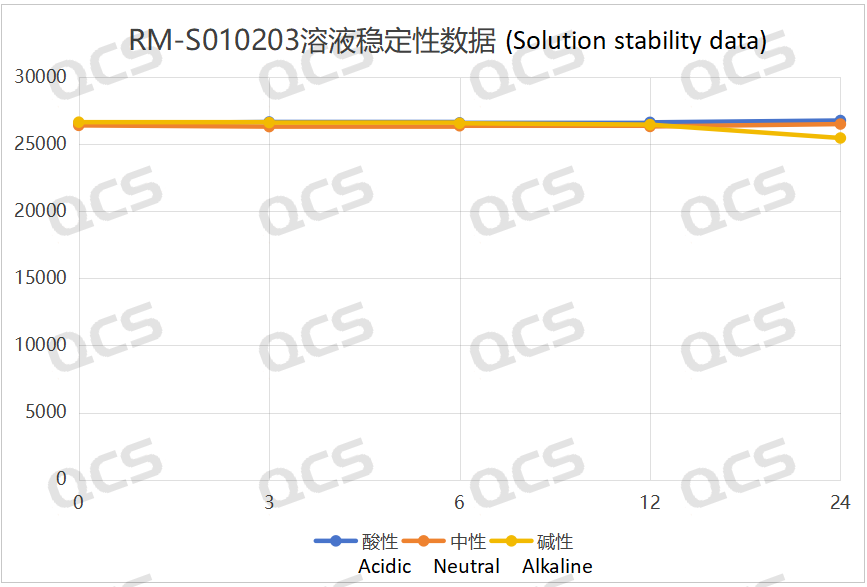
Figure 3: Summary of solution stability data of sample RM-S010203 (EP impurity C)
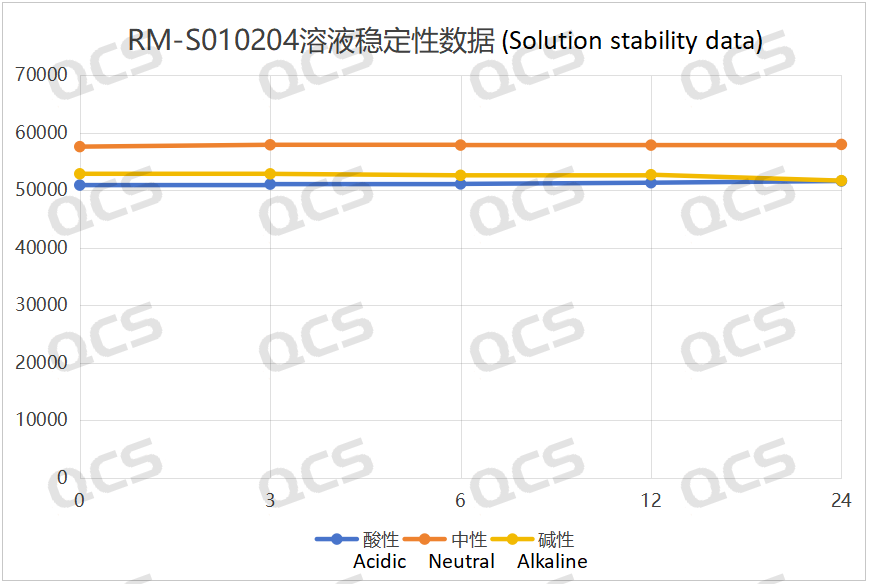
Figure 4: Summary of solution stability data for sample RM-S010204 (EP impurity D)
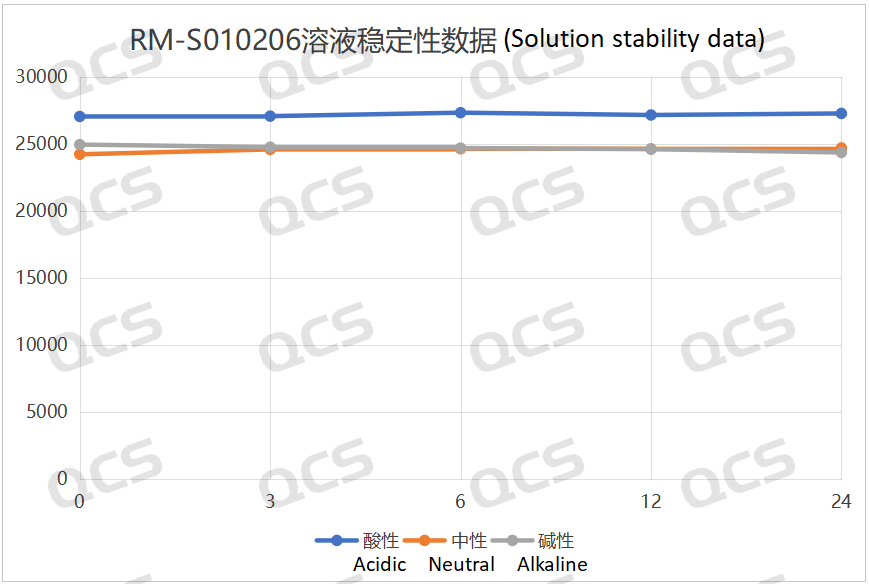
Figure 5: Summary of solution stability data for sample RM-S010206 (EP impurity F)
summary
In conclusion, through this experiment, we found that the samples RM-S010203 (EP impurity C), RM-S010204 (EP impurity D) and RM-S010206 (EP impurity F) have good stability in acidic, alkaline and neutral solutions. If customers need information on the stability of these three samples, they can consult our company.




Today, we share the study on the stability of specific impurities in short-acting β2-adrenergic receptor agonist—salbutamol. Salbutamol (salbutamol) is a short-acting β2-adrenergic receptor agonist used as an asthma reliever, effectively inhibiting the release of allergens such as histamine and preventing bronchial spasms. Adding trace amounts of salbutamol to livestock feed can increase lean meat yield and conversion rate, while reducing fat content. However, its toxicity is much higher than that of ractopamine, which has the same function. It is suitable for treating bronchial asthma, wheezing bronchitis, bronchial spasms, emphysema, and other conditions.
Experimental scheme
In this experiment, our center carried out a study on the solution stability of three specific impurities of salbutamol according to the chromatographic conditions used in item "Related substances" of "SALBUTAMOL" variety in European Pharmacopoeia 11.0, and the sample number and structural formula used are shown in Figure 1 and Figure 2:

Figure 1: The impurity number and structure of the impurity used in this study

Figure 2: Standard inclusion of impurity codes and the correspondence between impurity numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-S010203 (EP impurity C; Salbutamol EP Impurity C; CAS No:18910-68-4), RM-S010204 (EP impurity D; Salbutamol EP Impurity D; CAS No:156339-88-7), and RM-S010206 (EP impurity F; Salbutamol EP Impurity F; CAS No:147663-30-7). These samples were placed in acidic, neutral, and alkaline solutions, respectively. They were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours. Subsequently, the samples were analyzed using the chromatographic conditions specified under "Related substances" in the "SALBUTAMOL" section of the 11.0 edition of the European Pharmacopoeia. The changes in the main peak area of the chromatogram over time were observed to determine the stability of the sample solutions.
Experiment conclusion
After testing, it was found that the main peak area of samples RM-S010203 (EP impurity C), RM-S010204 (EP impurity D), and RM-S010206 (EP impurity F) did not change significantly over 24 hours in acidic, neutral, and alkaline solutions, with relative standard deviations all less than 2.0%. Therefore, these three samples can be considered relatively stable during the 24-hour period in acidic, neutral, and alkaline solutions. The main peak area data for each detection point under different pH conditions for samples RM-S010203 (EP impurity C), RM-S010204 (EP impurity D), and RM-S010206 (EP impurity F) are as follows:

Figure 3: Summary of solution stability data of sample RM-S010203 (EP impurity C)

Figure 4: Summary of solution stability data for sample RM-S010204 (EP impurity D)

Figure 5: Summary of solution stability data for sample RM-S010206 (EP impurity F)
summary
In conclusion, through this experiment, we found that the samples RM-S010203 (EP impurity C), RM-S010204 (EP impurity D) and RM-S010206 (EP impurity F) have good stability in acidic, alkaline and neutral solutions. If customers need information on the stability of these three samples, they can consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号