Time:2025-03-17
In drug development, the precise separation of chiral impurities is a core challenge in quality control. Recently, our technical team has solved the problem of the anaerobic adrenaline chiral compound separation in the central control analysis of the anaerobic epinephrine project! From literature reproduction to independent optimization, from "serious tail" to "perfect separation", let's take a look at the whole record of this technical breakthrough!
Product introduction: Deoxyepinephrine (Phenylephrine) is a α1 adrenergic receptor agonist, mainly used to constrict blood vessels and raise blood pressure. It mainly activates α1 receptors on vascular smooth muscle, causes vasoconstriction, increases peripheral resistance, and thus raises blood pressure.
1. Research and development background: the central control analysis encountered difficult problems
Since customers should focus on monitoring the amount of corresponding isomomer impurities when investigating anaerobic adrenaline, our center has also developed this chiral impurity. The specific product information is shown in Figure 1:
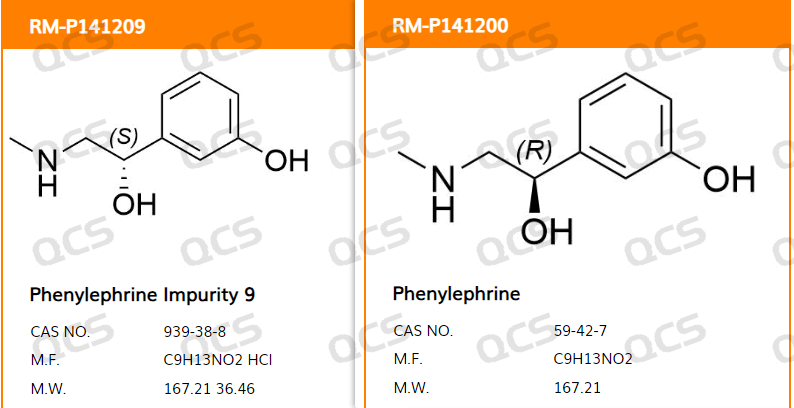
Figure 1: S-noradrenaline and adrenaline structural information
To ensure the smooth progress of the noxepinephrine project, the technical team should establish internal control detection methods for key chiral impurities. However, the separation effect of the target compound (deoxyepinephrine chiral isomer) has not reached the standard —— insufficient separation and exceeding the tail factor, which has become the "obstacle" on the research and development, as shown in Figure 2.
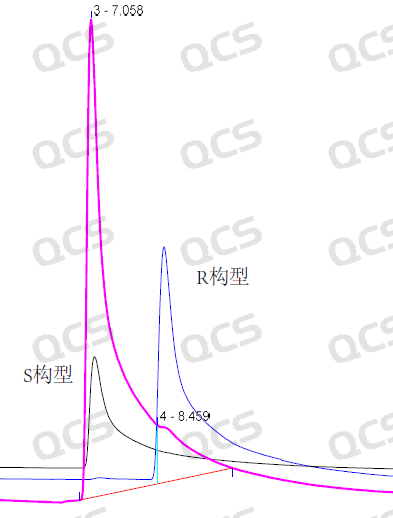
Figure 2: Initial test results
二、初探文献:方法复现遇瓶颈
2. Initial literature: method reproduction encountered a bottleneck
The team first adopted the customer sharing method (Figure 3) and configured the columns strictly according to the literature conditions (Figure 4:Dr.Maisch ReproSil Chiral-AM,5μm,250×4.6mm). However, the result are still not ideal, with the main peak and the impurity peak overlap seriously!

Figure 3: Customer sharing method

Figure 4: Customer chiral method chromatcolumn
三、自主优化:调整PH反遭“滑铁卢”
3. Independent optimization: adjust PH instead by "Waterloo"
Faced with the "acclimatization" of the literature method, the team tried to adjust the PH value of the mobile phase.
Acid modulation experiment: the main peak is seriously trailing;
Alkali modulation experiment: the impurity peak appears shoulder peak, and the separation degree decreases instead of rising (as shown in Figure 5).
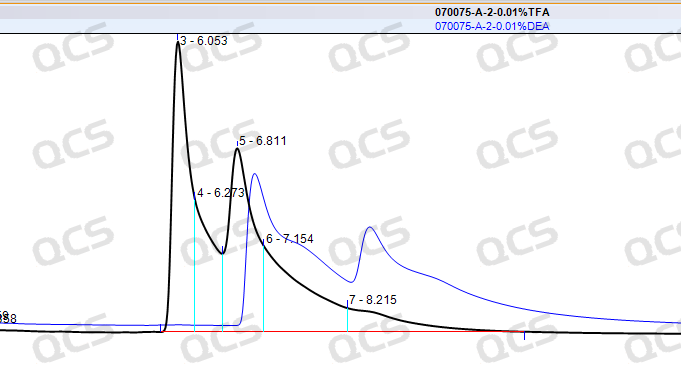
Figure 5: Chromatogram comparison after acid / alkali modulation
Conclusion: simply adjusting PH can not solve the problem, we need to find a new way!
4. The key to breaking the game: chromatography column screening "to the end"
The technical team carried out deep cooperation with a number of chiral column column manufacturers, screened the series of chiral column columns developed and created according to the chiral separation characteristics, and found that the Y3 column was the same as the column packing that provided the method.
Final solution: The research and creation brand chiral column (model: EnantioPak Y3250*4.6mm, 5um) stands out (see Figure 6)!
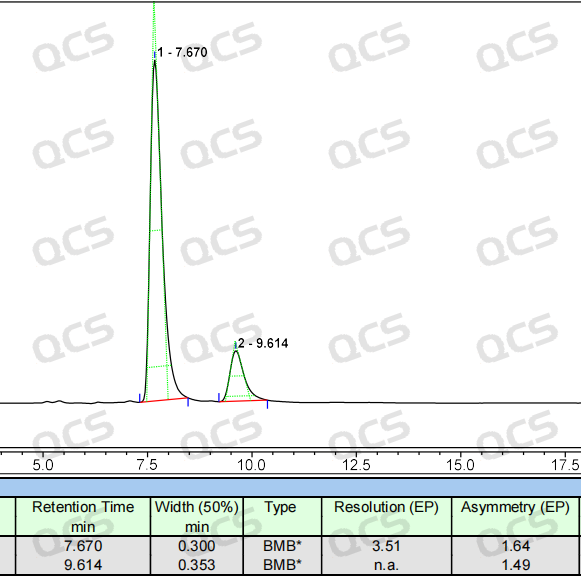
Figure 6: Optimized chromatogram
Amazing effect: the main peak and impurity peak baseline separation, peak shape sharp symmetry!、成果落地:内控方法终确
5. Results landing: the internal control method is finally established
Based on the optimized chromatographic conditions, the team successfully established the internal control and detection method for deoxyepinephrine chiral impurities, and the key parameters all met the requirements of project approval!
Peroration
From "failure of literature recovery" to "success of independent optimization", this technological battle not only reflects the team's research and development spirit of "fighting to the end", but also confirms the importance of accurate material selection and in-depth cooperation! In the future, we will continue to optimize the analysis method to ensure the drug quality! For technical details of support or cooperation discussion, please contact the company.

In drug development, the precise separation of chiral impurities is a core challenge in quality control. Recently, our technical team has solved the problem of the anaerobic adrenaline chiral compound separation in the central control analysis of the anaerobic epinephrine project! From literature reproduction to independent optimization, from "serious tail" to "perfect separation", let's take a look at the whole record of this technical breakthrough!
Product introduction: Deoxyepinephrine (Phenylephrine) is a α1 adrenergic receptor agonist, mainly used to constrict blood vessels and raise blood pressure. It mainly activates α1 receptors on vascular smooth muscle, causes vasoconstriction, increases peripheral resistance, and thus raises blood pressure.
1. Research and development background: the central control analysis encountered difficult problems
Since customers should focus on monitoring the amount of corresponding isomomer impurities when investigating anaerobic adrenaline, our center has also developed this chiral impurity. The specific product information is shown in Figure 1:

Figure 1: S-noradrenaline and adrenaline structural information
To ensure the smooth progress of the noxepinephrine project, the technical team should establish internal control detection methods for key chiral impurities. However, the separation effect of the target compound (deoxyepinephrine chiral isomer) has not reached the standard —— insufficient separation and exceeding the tail factor, which has become the "obstacle" on the research and development, as shown in Figure 2.

Figure 2: Initial test results
二、初探文献:方法复现遇瓶颈
2. Initial literature: method reproduction encountered a bottleneck
The team first adopted the customer sharing method (Figure 3) and configured the columns strictly according to the literature conditions (Figure 4:Dr.Maisch ReproSil Chiral-AM,5μm,250×4.6mm). However, the result are still not ideal, with the main peak and the impurity peak overlap seriously!

Figure 3: Customer sharing method

Figure 4: Customer chiral method chromatcolumn
三、自主优化:调整PH反遭“滑铁卢”
3. Independent optimization: adjust PH instead by "Waterloo"
Faced with the "acclimatization" of the literature method, the team tried to adjust the PH value of the mobile phase.
Acid modulation experiment: the main peak is seriously trailing;
Alkali modulation experiment: the impurity peak appears shoulder peak, and the separation degree decreases instead of rising (as shown in Figure 5).

Figure 5: Chromatogram comparison after acid / alkali modulation
Conclusion: simply adjusting PH can not solve the problem, we need to find a new way!
4. The key to breaking the game: chromatography column screening "to the end"
The technical team carried out deep cooperation with a number of chiral column column manufacturers, screened the series of chiral column columns developed and created according to the chiral separation characteristics, and found that the Y3 column was the same as the column packing that provided the method.
Final solution: The research and creation brand chiral column (model: EnantioPak Y3250*4.6mm, 5um) stands out (see Figure 6)!

Figure 6: Optimized chromatogram
Amazing effect: the main peak and impurity peak baseline separation, peak shape sharp symmetry!、成果落地:内控方法终确
5. Results landing: the internal control method is finally established
Based on the optimized chromatographic conditions, the team successfully established the internal control and detection method for deoxyepinephrine chiral impurities, and the key parameters all met the requirements of project approval!
Peroration
From "failure of literature recovery" to "success of independent optimization", this technological battle not only reflects the team's research and development spirit of "fighting to the end", but also confirms the importance of accurate material selection and in-depth cooperation! In the future, we will continue to optimize the analysis method to ensure the drug quality! For technical details of support or cooperation discussion, please contact the company.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号