Time:2025-02-13
Today, we will share a study on the stability of specific impurities in the new - type immunosuppressant tacrolimus. Tacrolimus, also known as FK506, is a fermentation product isolated from the genus Streptomyces. It is a macrolide drug and a potent new - type immunosuppressant. It mainly inhibits the release of interleukin - 2 (IL - 2) and comprehensively suppresses the function of T lymphocytes, being 100 times more potent than cyclosporine (CsA). In recent years, as a first - line drug for liver and kidney transplantation, it has been launched in 14 countries such as Japan and the United States. Clinical trials have shown that it has good efficacy in heart, lung, intestine, bone marrow and other transplants. Meanwhile, FK506 also plays an active role in the treatment of autoimmune diseases such as atopic dermatitis (AD), systemic lupus erythematosus (SLE), and autoimmune eye diseases.
Experimental scheme
In this experiment, our center referred to the chromatographic conditions under the “Related substances” section of the “TACROLIMUS MONOHYDRATE” monograph in the 11.0 edition of the European Pharmacopoeia to conduct a solution stability study on two specific impurities of tacrolimus. The catalog numbers and structural formulas of the samples used are shown in Figure 1 and Figure 2 below:
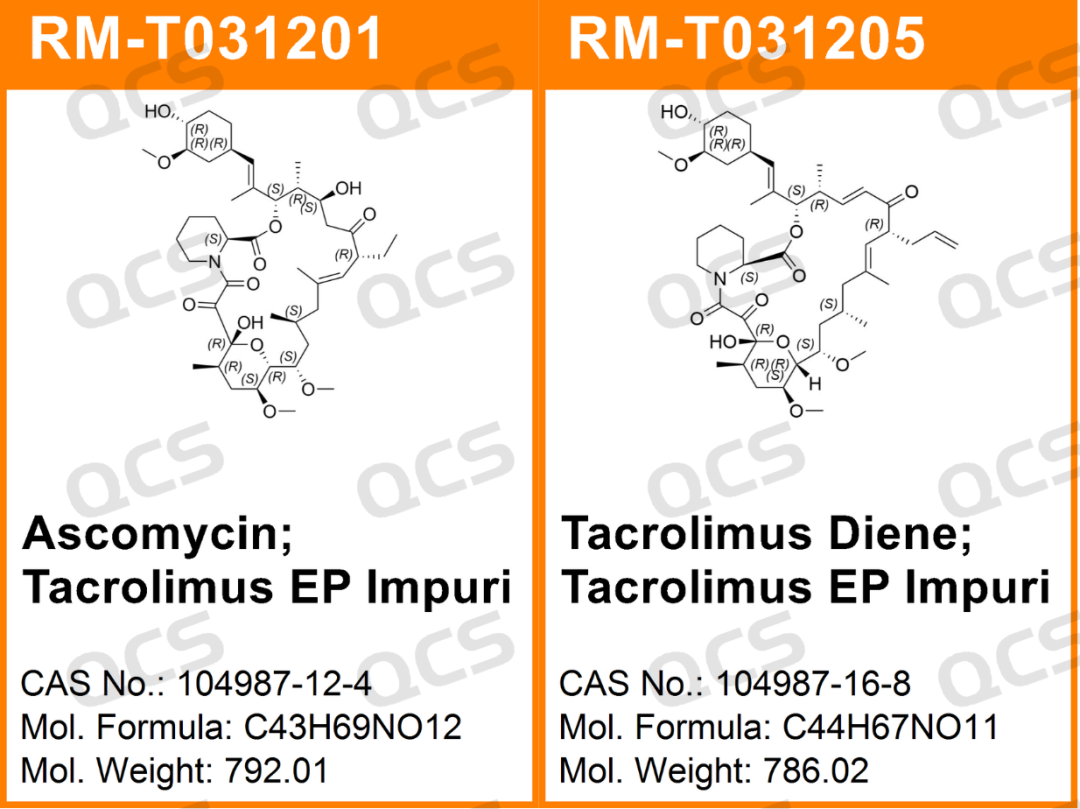
Figure 1: The impurity cargo number and structure type used in this study

Figure 2: The corresponding relation diagram between the impurity code included in the standard and the impurity cargo number used in this study
In this experiment, the experimenter took appropriate amounts of RM - T031201 (EP Impurity A; Ascomycin; Tacrolimus EP Impurity A; CAS NO: 104987 - 12 - 4) and RM - T031205 (EP Impurity I; Tacrolimus Diene; Tacrolimus EP Impurity I; CAS NO: 104987 - 16 - 8) respectively, and placed them in acidic, neutral, and alkaline solutions. After being stored at room temperature and normal pressure for 0, 3, 6, 12, and 24 hours respectively, the samples were injected for detection according to the chromatographic conditions under the “Related substances” section of the “TACROLIMUS MONOHYDRATE” monograph in the 11.0 edition of the European Pharmacopoeia. By observing the change in the peak area of the main peak in the chromatogram as the storage time of the sample solution increased, the solution stability of the samples was determined.
Experiment conclusion
After testing, it was found that for samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I), the peak areas of the main peaks did not change significantly during 24 - hour storage in acidic and neutral solutions, and the relative standard deviations were both less than 2.0%. Therefore, it can be considered that these two samples are relatively stable during 24 - hour storage in acidic and neutral solutions. However, it was also found through testing that the peak areas of the main peaks of samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I) changed significantly during 24 - hour storage in alkaline solutions, with relative standard deviations of 58.58% and 43.19% respectively. The experimenter summarized the sampling and testing data of the two samples during 24 - hour storage in acidic, neutral, and alkaline solutions, and created line graphs showing the change in the peak area of the main peak of the samples over time, as well as three - dimensional comparison graphs, as shown in Figures 3 - 6 below.
As can be seen from Figures 3 and 4, compared with the detection spectrum of sample RM - T031201 (EP Impurity A) stored in a neutral solution for 0 hours, the detection spectrum of this sample stored in an alkaline solution for 0 hours shows that the peak area of the main peak is much smaller, and there are other impurities. This indicates that the sample has degraded during the preparation process with the alkaline solution. Moreover, it can be seen from Figure 3 that sample RM - T031201 (EP Impurity A) degrades rapidly within 3 hours of storage in the alkaline solution, and after 3 hours of storage in the alkaline solution, the sample no longer degrades. From Figures 5 and 6, compared with the detection spectrum of sample RM - T031205 (EP Impurity I) stored in a neutral solution for 0 hours, the detection spectrum of this sample stored in an alkaline solution for 0 hours shows that the peak area of the main peak is much smaller, and there are other impurities. This indicates that the sample has degraded during the preparation process with the alkaline solution. It can be seen from Figure 5 that sample RM - T031205 (EP Impurity I) degrades rapidly within 3 hours of storage in the alkaline solution, and after 3 hours of storage in the alkaline solution, the sample will continue to degrade slowly.
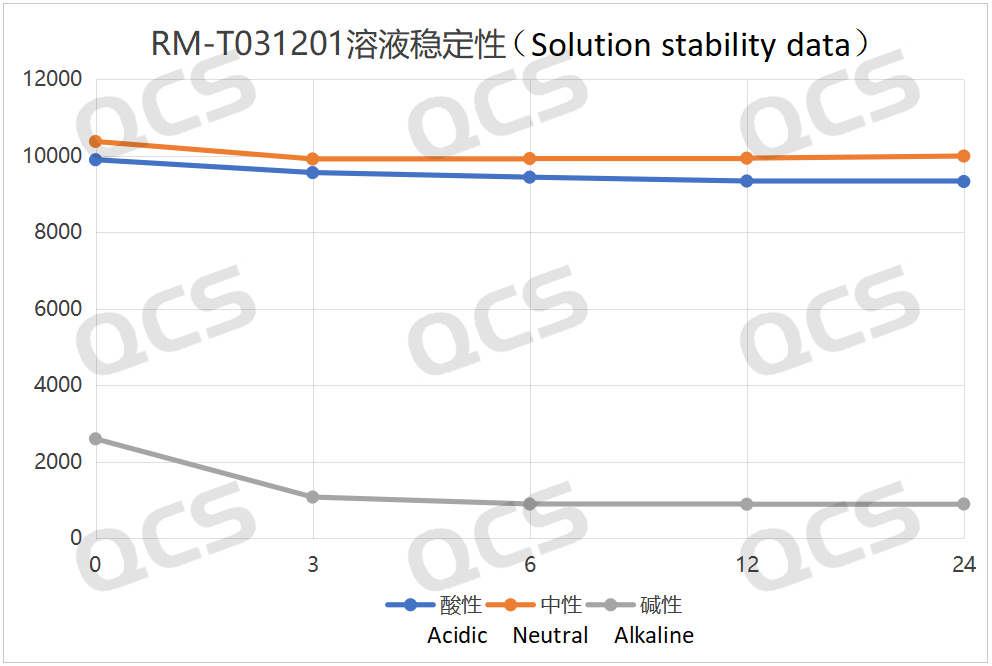
Figure 3: Summary line plot of solution stability data for sample RM-T031201 (EP impurity A) placed in acidic, neutral and alkaline solutions for 24 hours
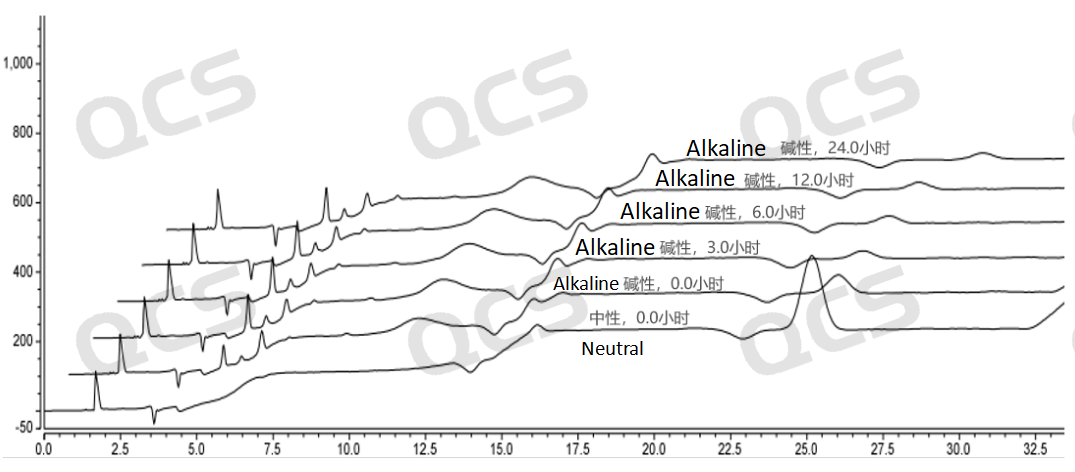
Figure 4: Stereo comparison of alkaline solution stability data for sample RM-T031201 (EP impurity A)
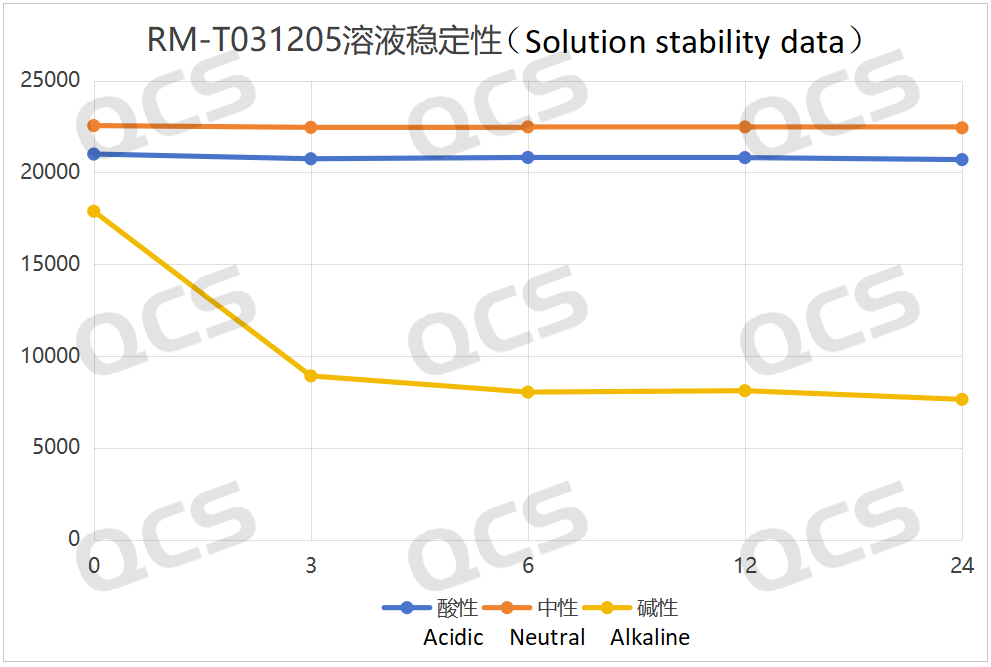
Figure 5: Summary line plot of solution stability data of sample RM-T031205 (EP impurity I) placed in acidic, neutral and alkaline for 24 hours
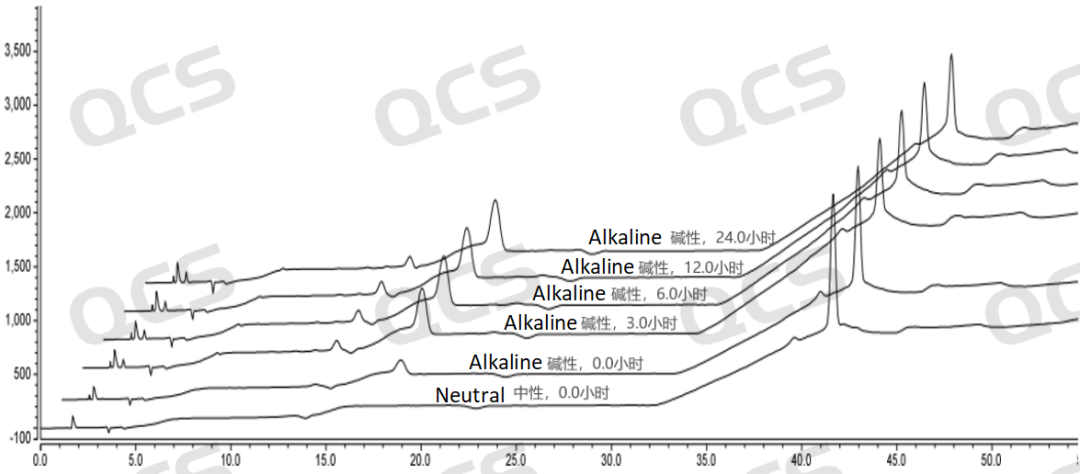
Figure 6: Stereo comparison of alkaline solution stability data of sample RM-T031205 (EP Impurity I)
summary
In conclusion, through this experiment, we found that samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I) have good stability in acidic and neutral solutions. These two samples will undergo partial degradation during the process of sample preparation with alkaline solutions and will continue to degrade during the detection process. Therefore, when customers detect samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I), they should avoid using alkaline diluents and should also prevent contact with alkalis during use, storage, and transportation. If customers need more information about the stability of these two samples, they can consult our company.

Today, we will share a study on the stability of specific impurities in the new - type immunosuppressant tacrolimus. Tacrolimus, also known as FK506, is a fermentation product isolated from the genus Streptomyces. It is a macrolide drug and a potent new - type immunosuppressant. It mainly inhibits the release of interleukin - 2 (IL - 2) and comprehensively suppresses the function of T lymphocytes, being 100 times more potent than cyclosporine (CsA). In recent years, as a first - line drug for liver and kidney transplantation, it has been launched in 14 countries such as Japan and the United States. Clinical trials have shown that it has good efficacy in heart, lung, intestine, bone marrow and other transplants. Meanwhile, FK506 also plays an active role in the treatment of autoimmune diseases such as atopic dermatitis (AD), systemic lupus erythematosus (SLE), and autoimmune eye diseases.
Experimental scheme
In this experiment, our center referred to the chromatographic conditions under the “Related substances” section of the “TACROLIMUS MONOHYDRATE” monograph in the 11.0 edition of the European Pharmacopoeia to conduct a solution stability study on two specific impurities of tacrolimus. The catalog numbers and structural formulas of the samples used are shown in Figure 1 and Figure 2 below:

Figure 1: The impurity cargo number and structure type used in this study

Figure 2: The corresponding relation diagram between the impurity code included in the standard and the impurity cargo number used in this study
In this experiment, the experimenter took appropriate amounts of RM - T031201 (EP Impurity A; Ascomycin; Tacrolimus EP Impurity A; CAS NO: 104987 - 12 - 4) and RM - T031205 (EP Impurity I; Tacrolimus Diene; Tacrolimus EP Impurity I; CAS NO: 104987 - 16 - 8) respectively, and placed them in acidic, neutral, and alkaline solutions. After being stored at room temperature and normal pressure for 0, 3, 6, 12, and 24 hours respectively, the samples were injected for detection according to the chromatographic conditions under the “Related substances” section of the “TACROLIMUS MONOHYDRATE” monograph in the 11.0 edition of the European Pharmacopoeia. By observing the change in the peak area of the main peak in the chromatogram as the storage time of the sample solution increased, the solution stability of the samples was determined.
Experiment conclusion
After testing, it was found that for samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I), the peak areas of the main peaks did not change significantly during 24 - hour storage in acidic and neutral solutions, and the relative standard deviations were both less than 2.0%. Therefore, it can be considered that these two samples are relatively stable during 24 - hour storage in acidic and neutral solutions. However, it was also found through testing that the peak areas of the main peaks of samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I) changed significantly during 24 - hour storage in alkaline solutions, with relative standard deviations of 58.58% and 43.19% respectively. The experimenter summarized the sampling and testing data of the two samples during 24 - hour storage in acidic, neutral, and alkaline solutions, and created line graphs showing the change in the peak area of the main peak of the samples over time, as well as three - dimensional comparison graphs, as shown in Figures 3 - 6 below.
As can be seen from Figures 3 and 4, compared with the detection spectrum of sample RM - T031201 (EP Impurity A) stored in a neutral solution for 0 hours, the detection spectrum of this sample stored in an alkaline solution for 0 hours shows that the peak area of the main peak is much smaller, and there are other impurities. This indicates that the sample has degraded during the preparation process with the alkaline solution. Moreover, it can be seen from Figure 3 that sample RM - T031201 (EP Impurity A) degrades rapidly within 3 hours of storage in the alkaline solution, and after 3 hours of storage in the alkaline solution, the sample no longer degrades. From Figures 5 and 6, compared with the detection spectrum of sample RM - T031205 (EP Impurity I) stored in a neutral solution for 0 hours, the detection spectrum of this sample stored in an alkaline solution for 0 hours shows that the peak area of the main peak is much smaller, and there are other impurities. This indicates that the sample has degraded during the preparation process with the alkaline solution. It can be seen from Figure 5 that sample RM - T031205 (EP Impurity I) degrades rapidly within 3 hours of storage in the alkaline solution, and after 3 hours of storage in the alkaline solution, the sample will continue to degrade slowly.

Figure 3: Summary line plot of solution stability data for sample RM-T031201 (EP impurity A) placed in acidic, neutral and alkaline solutions for 24 hours

Figure 4: Stereo comparison of alkaline solution stability data for sample RM-T031201 (EP impurity A)

Figure 5: Summary line plot of solution stability data of sample RM-T031205 (EP impurity I) placed in acidic, neutral and alkaline for 24 hours

Figure 6: Stereo comparison of alkaline solution stability data of sample RM-T031205 (EP Impurity I)
summary
In conclusion, through this experiment, we found that samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I) have good stability in acidic and neutral solutions. These two samples will undergo partial degradation during the process of sample preparation with alkaline solutions and will continue to degrade during the detection process. Therefore, when customers detect samples RM - T031201 (EP Impurity A) and RM - T031205 (EP Impurity I), they should avoid using alkaline diluents and should also prevent contact with alkalis during use, storage, and transportation. If customers need more information about the stability of these two samples, they can consult our company.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号