Time:2024-12-13
β-lactam antibiotics refer to a large class of antibiotics with a β-lactam ring in their chemical structure. They have poor chemical stability and often produce ring opening degradation impurities. This article will focus on the relevant research and data on β-lactam ring opening impurities.
Introduction to β-lactam antibiotics
β-lactam antibiotics refer to a large class of antibiotics with a β-lactam ring in their chemical structure, including the most commonly used penicillin and cephalosporins in clinical practice, as well as newly developed atypical β- lactam antibiotics such as cephalosporins, thiomycin, and monocyclic β- lactam antibiotics. This type of antibiotic has the advantages of strong bactericidal activity, low toxicity, wide indications, and good clinical efficacy. At present, generic drugs mainly involve several categories with similar structures, including cephalosporins, penicillin, and penems. The general classification is shown in Figure 1:
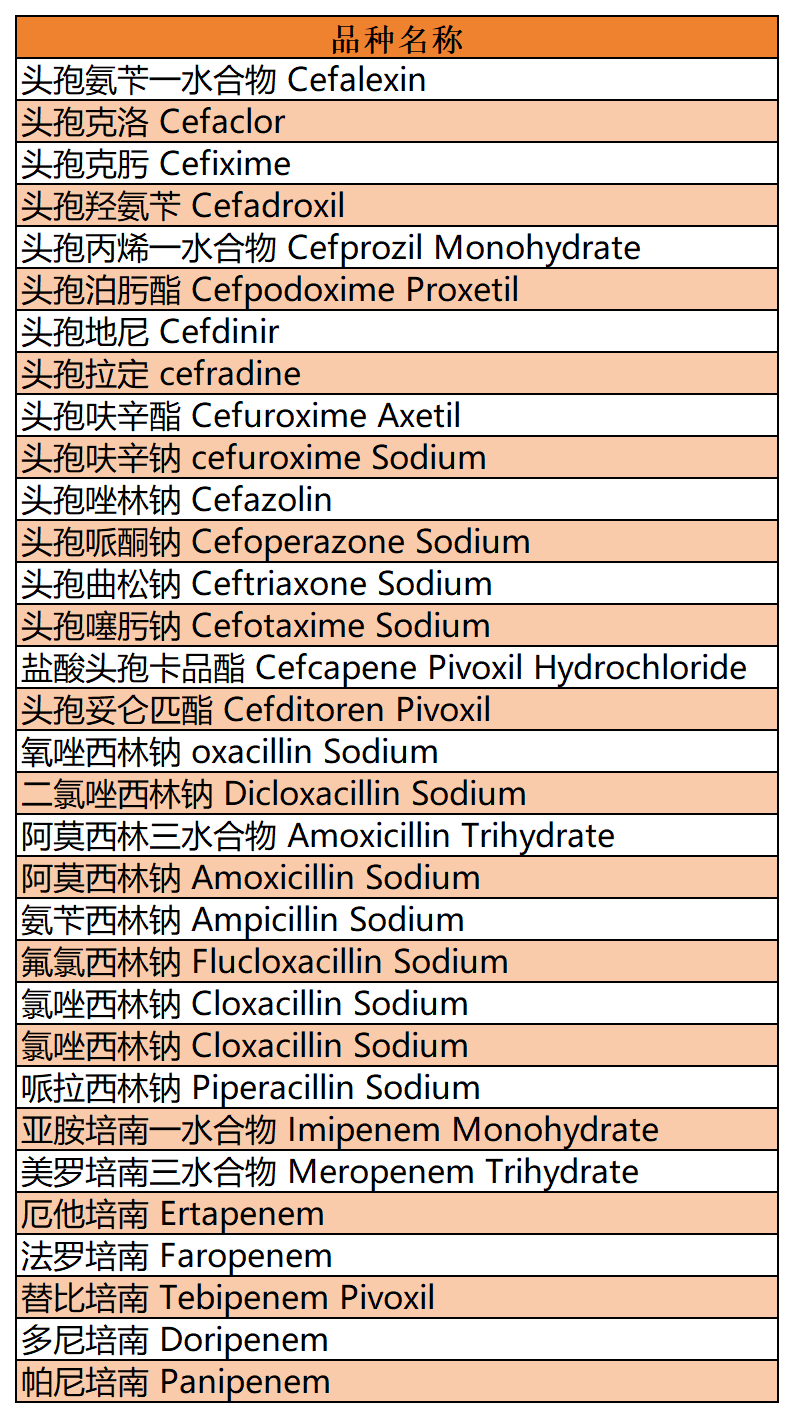
Figure 1: Classification of β-lactam antibiotics
Type and liquid phase peak situation after ring opening
Since the use of β-lactam antibiotics, scholars at home and abroad have unanimously believed that they are highly effective and less toxic antibiotics and are still widely used in clinical practice. During the synthesis and preparation of such drugs, it is sometimes inevitable to use conditions such as changing solvents, heating, altering pH, acidity or alkalinity. This will inevitably affect the stability of the β-lactam ring, and the products of its ring opening will bind with proteins to form antigens, leading to allergic reactions; In addition, the product after ring opening will undergo self polymerization during storage, and the higher the degree of polymerization, the stronger the allergic reaction.
By organizing the pharmacopoeia literature of the above varieties, it was found that impurities derived from ring opening or ring opening have been included in multiple pharmacopoeias. For example, some products contain β-lactam ring opening impurities (such as Amoxicillin, Piperacillin, Imipenem, etc.), lactam ring opening decarboxylation impurities (such as Amoxicillin, Cloxacillin, etc.), and lactam ring opening lactone impurities (such as Cefdinir). Liquid chromatography, as a commonly used analytical and detection method, is used for the identification of such impurities. However, we often face a practical problem of whether these ring opening impurities exhibit a single peak or multiple peaks under specified chromatographic conditions?
From the pharmacopoeial standards that can be found with records, the ring opening impurity of Meropenem is determined to be a single configuration (both EP and USP are included as single configuration-single peak), while other included ring opening impurities are basically assumed to be mixtures, except for a small portion of mixtures that exhibit single peak, such as the ring opening impurities of Cloxacillin Sodium and Cefazolin Sodium. Most of the other compounds exhibit multiple peaks (mostly bimodal) as ring opening mixtures, such as Amoxicillin ring opening impurities, Piperacillin ring opening impurities, Oxacillin Sodium ring opening impurities, Diclofacillin Sodium ring opening impurities, Flucloxacillin Sodium ring opening impurities, Cloxacillin Sodium ring opening impurities, and Imipenem ring opening impurities, all of which are explicitly listed as mixtures and bimodal products by EP or USP. The specific product related information is organized as shown in Figure 2:
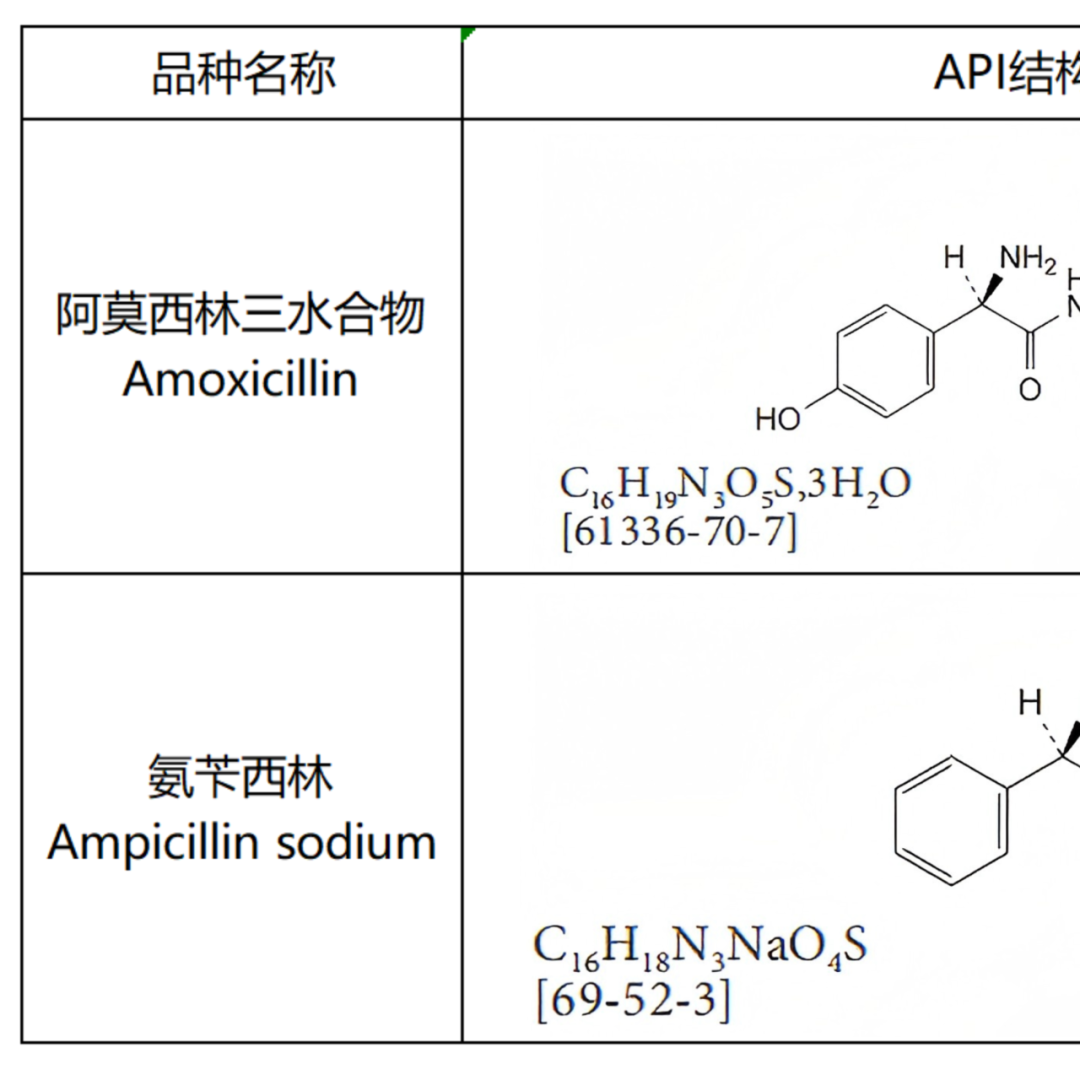
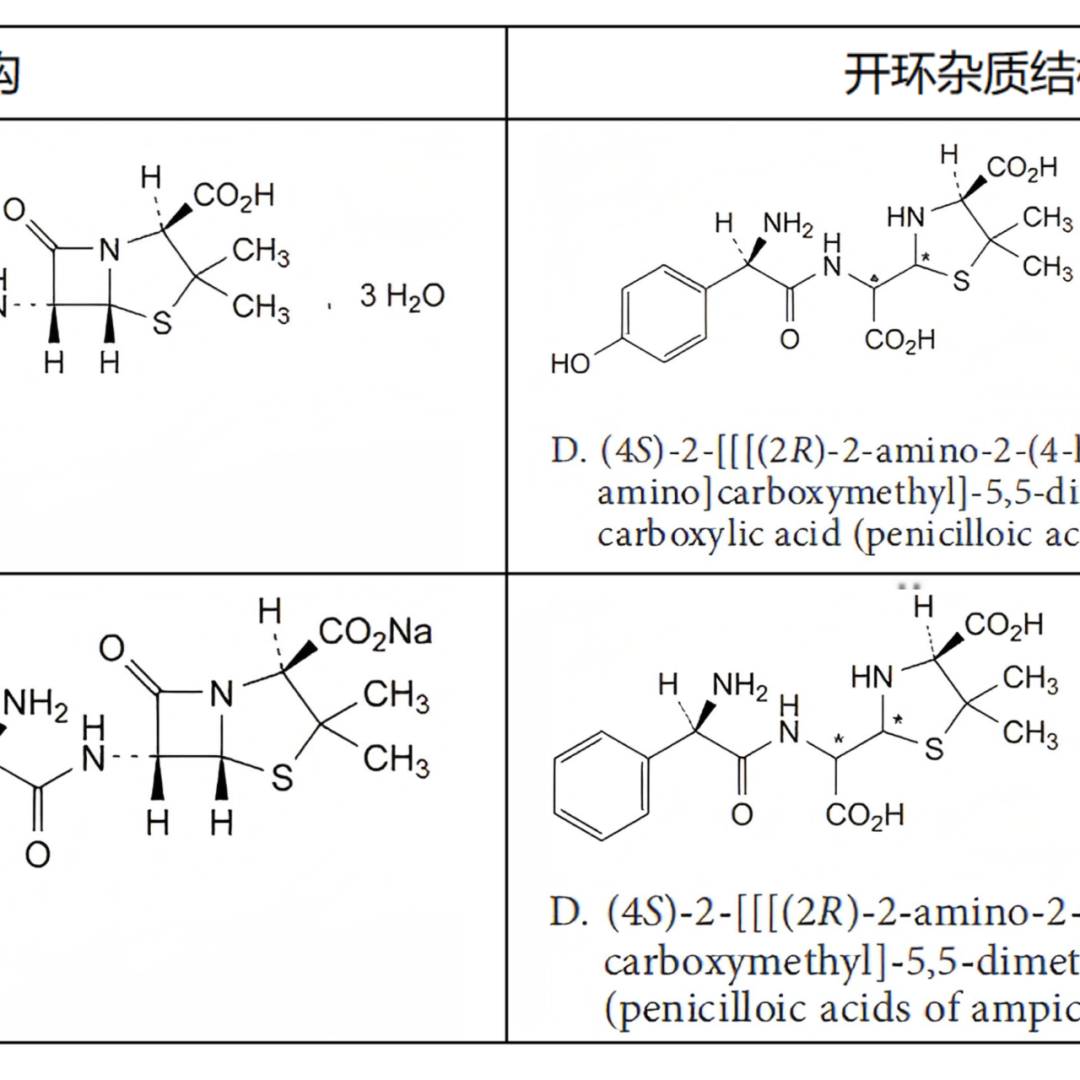
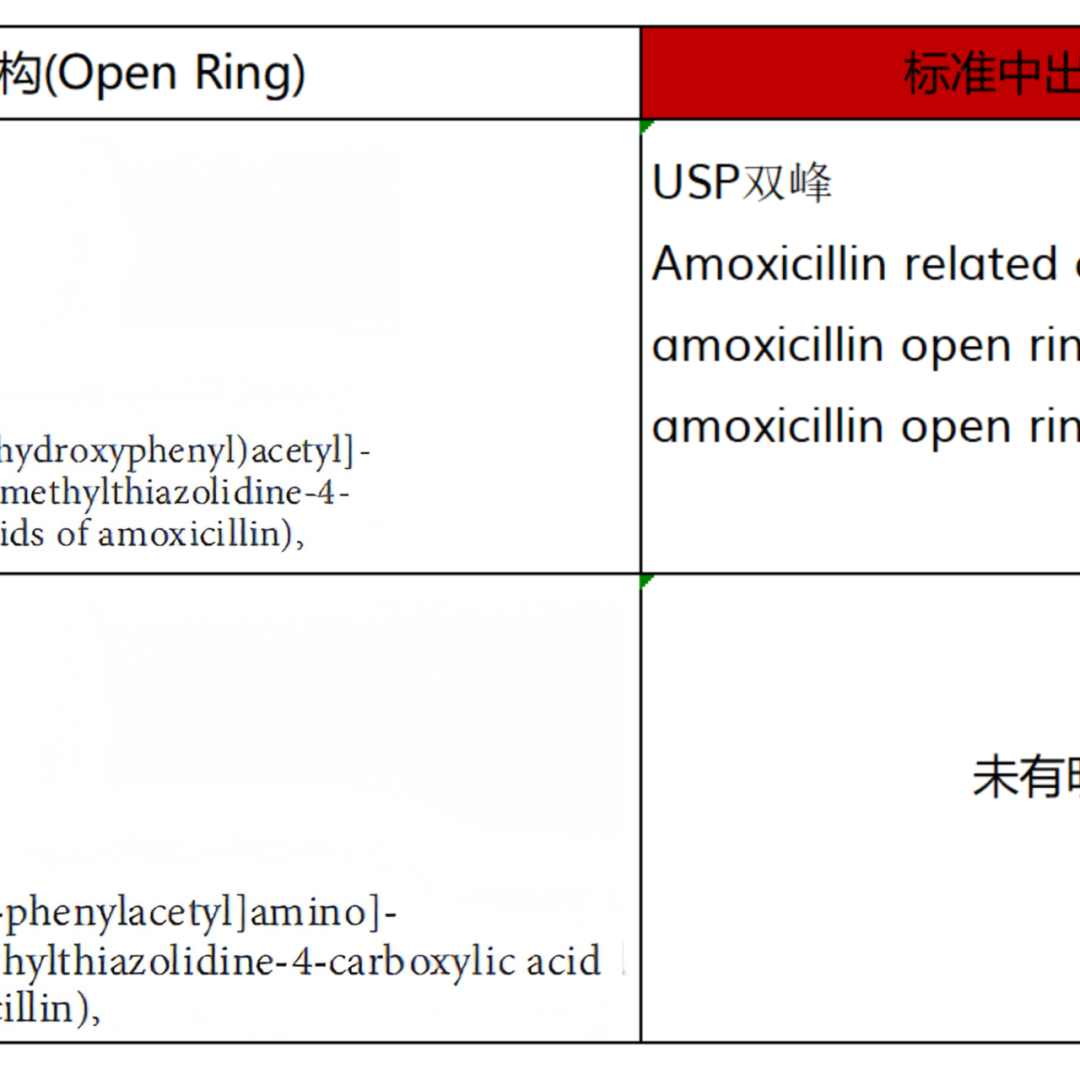
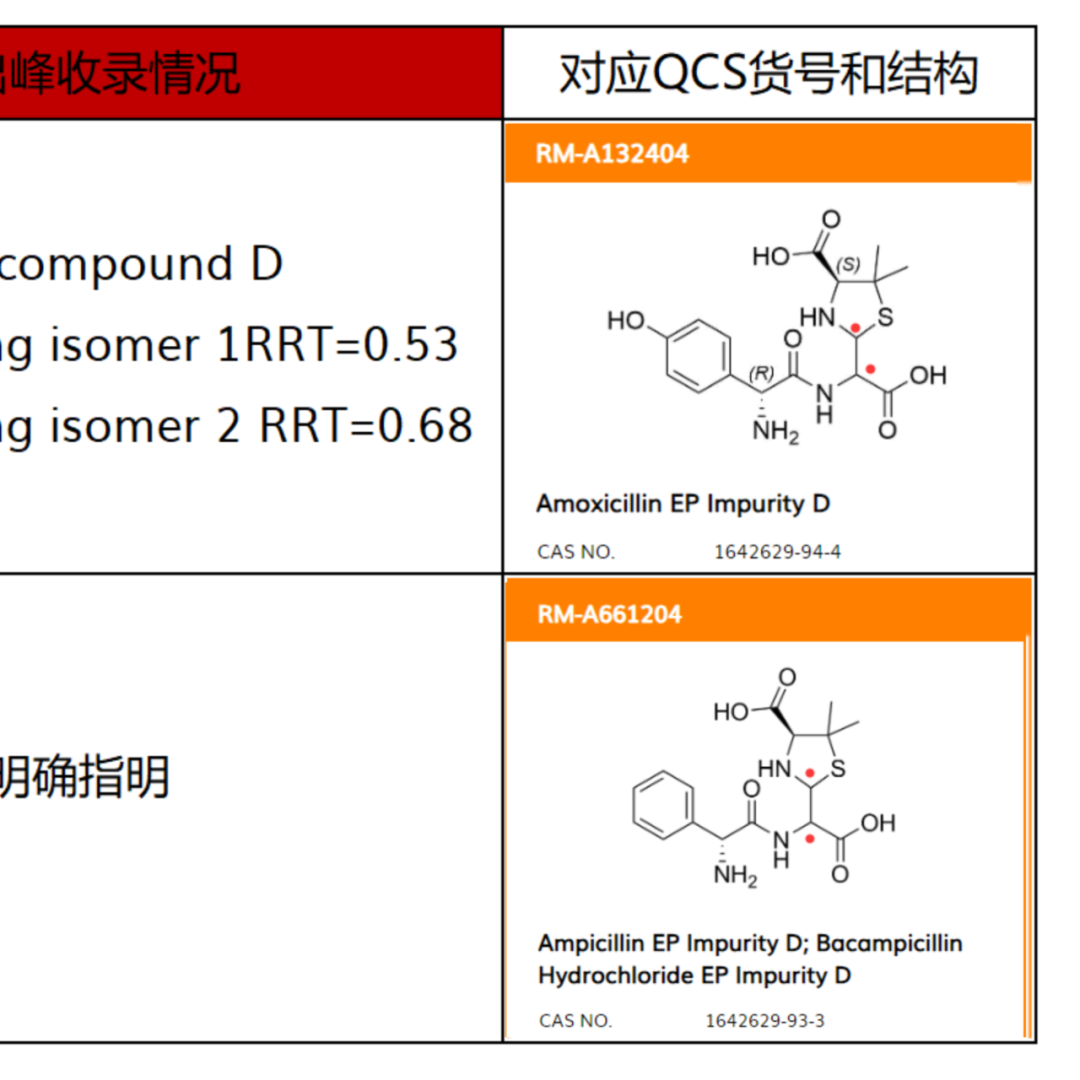
Figure 2: Summary table of β-lactam Ring Opening Impurities structure and peak appearance
Discussion: Ring opening impurities of cefditoren pivoxil
In this issue, we would like to discuss whether the ring opening impurity of cefditoren pivoxil is a single peak or a double peak. The P1 impurity in the import registration standard is not limited to a single chiral configuration structure. The chemical name of the P1 impurity only specifies the double bond configuration. From the standard, it can be confirmed that the ring opening product is a mixture, that is, whether it is a single chirality or a chiral center racemization after ring opening. At present, it cannot be confirmed. The structure of the P1 product is shown in Figure 3:
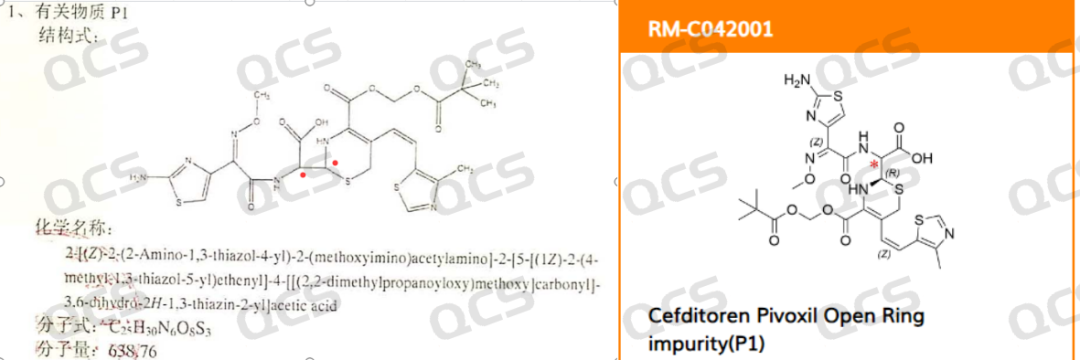
Figure 3: Import standards and QCS website inclusion of P1 ring opening impurity in cefditoren pivoxil
By simulating the actual degradation conditions of the drug, according to our current experimental results, it is basically impossible to obtain a single configuration of the product from the ring opening of cefditoren pivoxil; No matter what ring opening conditions are used (such as sodium hydroxide, lithium hydroxide, sodium bicarbonate, etc.), when combined with several of our 01 detection data, they all show a bimodal pattern. Secondly, we have also tried to prepare a single peak separately, but there will still be a small amount of isomers produced after freeze-drying the product. From the relevant substance items in the import registration standard (JX20090123) for cefditoren pivoxil granules, P1 is not a single peak, but rather a double peak. However, the standard is too vague and only provides a range of retention times, as shown in Figure 4:
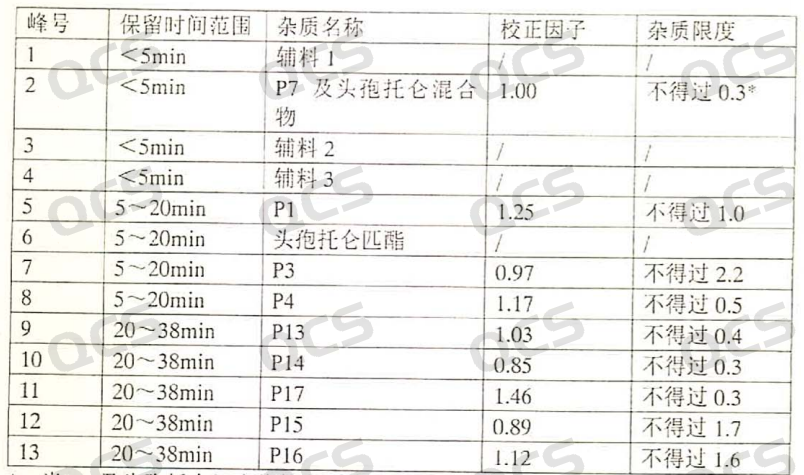
Figure 4: Relevant substance items for the import standard of cefditoren pivoxil granules
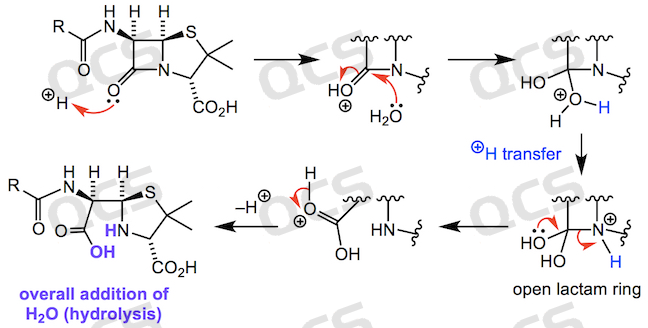
As shown in the above figure, under acidic or alkaline conditions, the carbonyl group of β - lactam is attacked by nucleophiles and undergoes ring opening reaction, while the adjacent chiral center undergoes chiral reversal under catalytic conditions to form a mixture structure. Due to the influence of active functional groups in drug molecules, chiral racemization reactions are prone to occur during the ring opening process.
In addition, the import standard mentions ring opening impurities (P1), ring opening dimers (P16), and dipile ester impurities (P17) (as shown in Figure 5), all of which are by-products of ring opening or ring opening. If it is difficult to obtain monomers from ring opening dimers, the probability of obtaining monomers from ring opening impurities as by-products can be imagined. In addition, from our degradation conditions for ring opening impurities, it is basically difficult to control to only obtain monomers. Currently, we have encountered many customers who require a single peak for ring opening impurities. We have also prepared and separated the ring opening impurities, and separated the single peak ring opening impurities. However, from the perspective of quality research, the product produced an ring opening during acceleration or long-term stability, but only two peaks (we have conducted relevant strong degradation results). If we previously studied the ring opening as a single peak, it will inevitably result in the production of a new and large unknown impurity. How to qualitatively and supplement research in the future, this issue may require special attention from R&D teachers. If the ring opening impurities are like this, then the ring opening dimer (P16) and ring opening dipile ester (P17) impurities will be very clear.
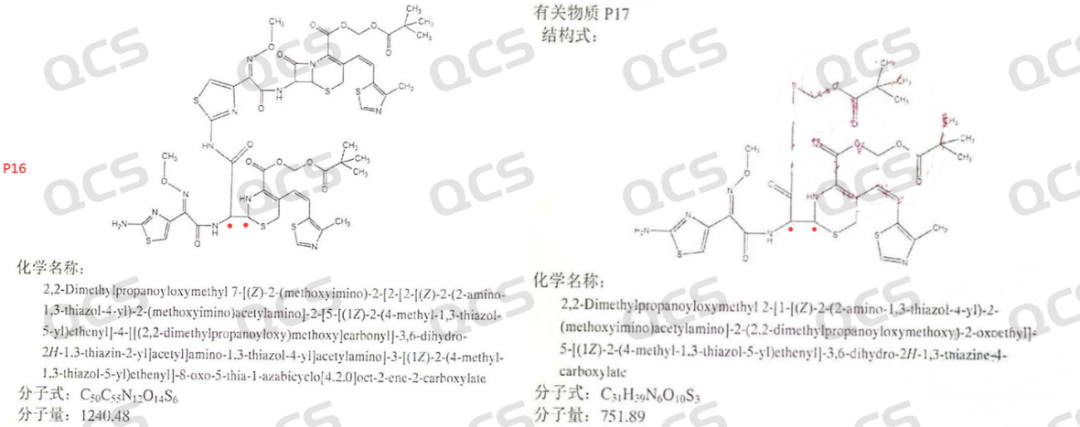
Figure 5: Structural information of substances P16 and P17 related to the import standard of cefditoren pivoxil granules
Finally, a summary of the collected information was compiled. If you are interested, please contact us for further information (as shown in Figure 6).
Figure 6: Summary of pharmacopoeia standards for β-lactam antibiotics

β-lactam antibiotics refer to a large class of antibiotics with a β-lactam ring in their chemical structure. They have poor chemical stability and often produce ring opening degradation impurities. This article will focus on the relevant research and data on β-lactam ring opening impurities.
Introduction to β-lactam antibiotics
β-lactam antibiotics refer to a large class of antibiotics with a β-lactam ring in their chemical structure, including the most commonly used penicillin and cephalosporins in clinical practice, as well as newly developed atypical β- lactam antibiotics such as cephalosporins, thiomycin, and monocyclic β- lactam antibiotics. This type of antibiotic has the advantages of strong bactericidal activity, low toxicity, wide indications, and good clinical efficacy. At present, generic drugs mainly involve several categories with similar structures, including cephalosporins, penicillin, and penems. The general classification is shown in Figure 1:

Figure 1: Classification of β-lactam antibiotics
Type and liquid phase peak situation after ring opening
Since the use of β-lactam antibiotics, scholars at home and abroad have unanimously believed that they are highly effective and less toxic antibiotics and are still widely used in clinical practice. During the synthesis and preparation of such drugs, it is sometimes inevitable to use conditions such as changing solvents, heating, altering pH, acidity or alkalinity. This will inevitably affect the stability of the β-lactam ring, and the products of its ring opening will bind with proteins to form antigens, leading to allergic reactions; In addition, the product after ring opening will undergo self polymerization during storage, and the higher the degree of polymerization, the stronger the allergic reaction.
By organizing the pharmacopoeia literature of the above varieties, it was found that impurities derived from ring opening or ring opening have been included in multiple pharmacopoeias. For example, some products contain β-lactam ring opening impurities (such as Amoxicillin, Piperacillin, Imipenem, etc.), lactam ring opening decarboxylation impurities (such as Amoxicillin, Cloxacillin, etc.), and lactam ring opening lactone impurities (such as Cefdinir). Liquid chromatography, as a commonly used analytical and detection method, is used for the identification of such impurities. However, we often face a practical problem of whether these ring opening impurities exhibit a single peak or multiple peaks under specified chromatographic conditions?
From the pharmacopoeial standards that can be found with records, the ring opening impurity of Meropenem is determined to be a single configuration (both EP and USP are included as single configuration-single peak), while other included ring opening impurities are basically assumed to be mixtures, except for a small portion of mixtures that exhibit single peak, such as the ring opening impurities of Cloxacillin Sodium and Cefazolin Sodium. Most of the other compounds exhibit multiple peaks (mostly bimodal) as ring opening mixtures, such as Amoxicillin ring opening impurities, Piperacillin ring opening impurities, Oxacillin Sodium ring opening impurities, Diclofacillin Sodium ring opening impurities, Flucloxacillin Sodium ring opening impurities, Cloxacillin Sodium ring opening impurities, and Imipenem ring opening impurities, all of which are explicitly listed as mixtures and bimodal products by EP or USP. The specific product related information is organized as shown in Figure 2:




Figure 2: Summary table of β-lactam Ring Opening Impurities structure and peak appearance
Discussion: Ring opening impurities of cefditoren pivoxil
In this issue, we would like to discuss whether the ring opening impurity of cefditoren pivoxil is a single peak or a double peak. The P1 impurity in the import registration standard is not limited to a single chiral configuration structure. The chemical name of the P1 impurity only specifies the double bond configuration. From the standard, it can be confirmed that the ring opening product is a mixture, that is, whether it is a single chirality or a chiral center racemization after ring opening. At present, it cannot be confirmed. The structure of the P1 product is shown in Figure 3:

Figure 3: Import standards and QCS website inclusion of P1 ring opening impurity in cefditoren pivoxil
By simulating the actual degradation conditions of the drug, according to our current experimental results, it is basically impossible to obtain a single configuration of the product from the ring opening of cefditoren pivoxil; No matter what ring opening conditions are used (such as sodium hydroxide, lithium hydroxide, sodium bicarbonate, etc.), when combined with several of our 01 detection data, they all show a bimodal pattern. Secondly, we have also tried to prepare a single peak separately, but there will still be a small amount of isomers produced after freeze-drying the product. From the relevant substance items in the import registration standard (JX20090123) for cefditoren pivoxil granules, P1 is not a single peak, but rather a double peak. However, the standard is too vague and only provides a range of retention times, as shown in Figure 4:

Figure 4: Relevant substance items for the import standard of cefditoren pivoxil granules

As shown in the above figure, under acidic or alkaline conditions, the carbonyl group of β - lactam is attacked by nucleophiles and undergoes ring opening reaction, while the adjacent chiral center undergoes chiral reversal under catalytic conditions to form a mixture structure. Due to the influence of active functional groups in drug molecules, chiral racemization reactions are prone to occur during the ring opening process.
In addition, the import standard mentions ring opening impurities (P1), ring opening dimers (P16), and dipile ester impurities (P17) (as shown in Figure 5), all of which are by-products of ring opening or ring opening. If it is difficult to obtain monomers from ring opening dimers, the probability of obtaining monomers from ring opening impurities as by-products can be imagined. In addition, from our degradation conditions for ring opening impurities, it is basically difficult to control to only obtain monomers. Currently, we have encountered many customers who require a single peak for ring opening impurities. We have also prepared and separated the ring opening impurities, and separated the single peak ring opening impurities. However, from the perspective of quality research, the product produced an ring opening during acceleration or long-term stability, but only two peaks (we have conducted relevant strong degradation results). If we previously studied the ring opening as a single peak, it will inevitably result in the production of a new and large unknown impurity. How to qualitatively and supplement research in the future, this issue may require special attention from R&D teachers. If the ring opening impurities are like this, then the ring opening dimer (P16) and ring opening dipile ester (P17) impurities will be very clear.

Figure 5: Structural information of substances P16 and P17 related to the import standard of cefditoren pivoxil granules
Finally, a summary of the collected information was compiled. If you are interested, please contact us for further information (as shown in Figure 6).

Figure 6: Summary of pharmacopoeia standards for β-lactam antibiotics

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号